Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2017-11-16 , DOI: 10.1016/j.bioorg.2017.11.010 Mohd. Javed Naim , Ozair Alam , Md. Jahangir Alam , Md. Quamrul Hassan , Nadeem Siddiqui , V.G.M. Naidu , Md. Iqbal Alam
|
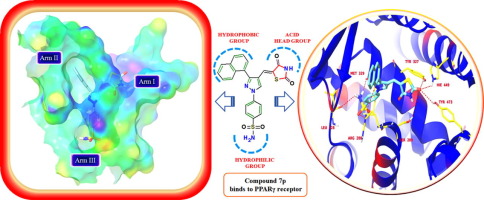
We herein report the design, synthesis and molecular docking studies of 2,4-thiazolidinedione derivatives containing benzene sulphonyl group which are docked against the Peroxisome Proliferator Activated Receptor (PPARγ) target. Compound 7p was most effective in lowering the blood glucose level as compared to standard drugs pioglitazone and rosiglitazone. Compound 7p exhibited potent PPAR-γ transactivation of 61.2% with 1.9 folds increase in gene expression. In molecular docking studies 7p showed excellent interactions with amino acids TYR 473, SER 289, HIE 449, TYR 327, ARG 288, MET 329 and LEU 228. Compound 7p did not cause any damage to the liver without any noteworthy weight gain and may be considered as promising candidates for the development of new antidiabetic agents.
中文翻译:

以吡唑核为潜在抗糖尿病药的噻唑烷二酮基苯磺酰胺衍生物的设计,合成和分子对接
我们在此报告了针对过氧化物酶体增殖物激活受体(PPARγ)靶标的含苯磺酰基的2,4-噻唑烷二酮衍生物的设计,合成和分子对接研究。与标准药物吡格列酮和罗格列酮相比,化合物7p在降低血糖水平方面最有效。化合物7p表现出61.2%的有效PPAR-γ反式激活,基因表达增加1.9倍。在分子对接研究中,7p显示出与氨基酸TYR 473,SER 289,HIE 449,TYR 327,ARG 288,MET 329和LEU 228的出色相互作用。化合物7p 不会对肝脏造成任何损害,而不会引起任何明显的体重增加,因此可以被视为开发新的抗糖尿病药物的有希望的候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号