Catalysis Communications ( IF 3.4 ) Pub Date : 2017-12-08 , DOI: 10.1016/j.catcom.2017.12.004 Haibiao Yu , Xinping Wang
|
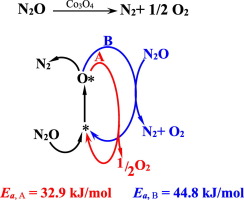
Apparent activation energies and reaction rates of N2O decomposition via L-H and E-R routes over Co3O4 were experimentally estimated after considering the increase of active sites with reaction temperature. It was found that the apparent activation energy is 32.9 kJ/mol for the L-H route and 44.8 kJ/mol for the E-R route, while the order of reaction with respect to O2 is − 0.367 at 350 °C and − 0.338 at 450 °C. The contribution of the E-R route to the total reaction rate was found to be considerably increased with the reaction temperature and which was well interpreted by the apparent activation energies of the two reaction routes.
中文翻译:

在Co 3 O 4上通过不同途径分解的N 2 O的表观活化能和反应速率
考虑到活性位点随反应温度的增加,通过实验估算了表观活化能和通过LH和ER途径在Co 3 O 4上分解N 2 O的反应速率。发现LH途径的表观活化能为32.9 kJ / mol,ER途径的表观活化能为44.8 kJ / mol,而相对于O 2的反应顺序在350°C为-0.367,在450°为-0.338。 C。发现ER途径对总反应速率的贡献随着反应温度而显着增加,并且这可以由两条反应途径的表观活化能很好地解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号