Current Opinion in Colloid & Interface Science ( IF 7.9 ) Pub Date : 2017-09-23 , DOI: 10.1016/j.cocis.2017.09.001 Aijaz Ahmad Dar , Carlos Bravo-Diaz , Nighat Nazir , Laurence Stuart Romsted
|
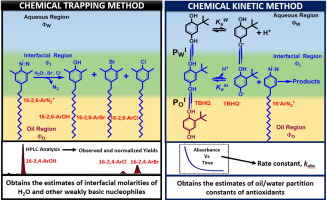
Two problems in colloid chemistry have proved difficult to solve. First the specific counterion and hydration effects on the properties of association colloids of ionic surfactants such as micelles, microemulsions and vesicles and second the efficiency of antioxidants, AOs, in fluid, opaque, usually nonionic emulsions. Specific ion and hydration effects are important because they contribute to the balance-of-forces determining association colloid properties along with the hydrophobic effect, however, few methods provide estimates of interfacial molarities. Determining antioxidant efficiencies in emulsions, an important problem in food science, is difficult because emulsions are opaque and antioxidant (AO) distributions are difficult to monitor. This review reports the development of two methods: chemical trapping (CT) and chemical kinetic (CK) methods for solving these problems, both based on the chemistries of arenediazonium ions. The CT method provides estimates of interfacial molarities of weakly basic nucleophiles including water, halides, alkyl carboxylates and sulfates, alcohols (including nonionic surfactants) and amide oxygen in the interfacial regions of association colloids. Results to date show that specific ion effects on association colloid properties, such as the sphere-to-rod transition concentration also involve displacement of interfacial water. The CK method, provides the observed rate constant, kobs, described by a pseudophase kinetic model for the reduction of the arenediazonium ion by an AO in intact emulsions to determine the oil/water partition constants by fitting kinetic data with two equations in two unknowns to obtain estimates of distributions and concentrations of AOs in the oil, interfacial and aqueous regions of nonionic emulsions. Part I of this review describes new insights obtained by the chemical trapping CT method, especially how changes in interfacial water and counterion molarities depend on their bulk solution concentrations. Part II reviews the chemical kinetic CK method based on the pseudophase kinetic model for determining the distributions of AOs in nonionic surfactant emulsions and provides a natural explanation for the cut-off effect. Part III describes a new combined application of the CT and CK methods for interpreting the complex kinetics of TBHQ reduction by the arenediazonium ion in mixed cationic/nonionic micelles. Perspectives and prospects complete the review in Part IV.
中文翻译:

化学动力学和化学捕获方法:分别测定缔合胶体中水,抗衡阴离子和其他弱碱性亲核试剂的抗氧化剂分布和界面摩尔浓度的独特方法
胶体化学中的两个问题已被证明难以解决。首先,特定的抗衡离子和水合作用对离子表面活性剂(例如微团,微乳液和囊泡)的缔合胶体的性能产生影响;其次,在流体,不透明(通常为非离子型)乳液中,抗氧化剂,AO的效率较高。特定的离子和水合作用很重要,因为它们有助于确定力,从而决定缔合胶体的性质以及疏水作用,但是,很少有方法提供界面摩尔浓度的估算值。确定乳液中的抗氧化剂效率是食品科学中的一个重要问题,这很困难,因为乳液是不透明的,并且抗氧化剂(AO)的分布很难监控。这篇评论报告了两种方法的发展:化学捕集(CT)和化学动力学(CK)方法来解决这些问题,这两种方法均基于槟榔重氮离子的化学性质。CT方法提供了缔合胶体界面区域中弱碱性亲核试剂(包括水,卤化物,烷基羧酸盐和硫酸盐,醇(包括非离子表面活性剂)和酰胺氧)的界面摩尔浓度的估计值。迄今为止的结果表明,特定的离子对缔合胶体性质的影响(例如,球至棒的过渡浓度)也涉及界面水的置换。CK方法可提供观察到的速率常数,在缔合胶体的界面区域中的烷基羧酸盐和硫酸盐,醇(包括非离子表面活性剂)和酰胺氧。迄今为止的结果表明,特定的离子对缔合胶体性质的影响(例如,球至棒的过渡浓度)也涉及界面水的置换。CK方法可提供观察到的速率常数,在缔合胶体的界面区域中的烷基羧酸盐和硫酸盐,醇(包括非离子表面活性剂)和酰胺氧。迄今为止的结果表明,特定的离子对缔合胶体性质的影响(例如,球至棒的过渡浓度)也涉及界面水的置换。CK方法可提供观察到的速率常数,k obs,由伪相动力学模型描述,用于通过完整乳液中的AO还原AO还原二氮杂zon离子,以确定油/水分配常数,方法是将动力学数据与两个未知数中的两个方程拟合,以获取AO中AO的分布和浓度的估计值非离子乳液的油,界面和水区域。这篇综述的第一部分描述了通过化学捕集CT方法获得的新见解,特别是界面水和抗衡离子摩尔浓度的变化如何取决于它们的整体溶液浓度。第二部分综述了基于伪相动力学模型的化学动力学CK方法,用于确定非离子表面活性剂乳液中AO的分布,并为截止效应提供了自然的解释。第三部分介绍了CT和CK方法的新组合应用,用于解释在混合的阳离子/非离子胶束中苯二氮杂离子还原TBHQ的复杂动力学。观点和前景完成了第四部分的审查。











































 京公网安备 11010802027423号
京公网安备 11010802027423号