Acta Biomaterialia ( IF 9.4 ) Pub Date : 2017-12-06 , DOI: 10.1016/j.actbio.2017.11.048 Giyeong Son , Byung Il Lee , You Jung Chung , Chan Beum Park
|
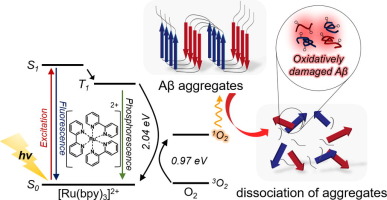
The self-assembly of β-amyloid (Aβ) peptides into highly stable plaques is a major hallmark of Alzheimer's disease. Here, we report visible light-driven dissociation of β-sheet-rich Aβ aggregates into small, nontoxic fragments using ruthenium (II) complex {[Ru(bpy)3]2+} that functions as a highly sensitive, biocompatible, photoresponsive anti-Aβ agent. According to our multiple analyses using thioflavin T, bicinchoninic acid, dynamic light scattering, atomic force microscopy, circular dichroism, and Fourier transform infrared spectroscopy, [Ru(bpy)3]2+ successfully disassembled Aβ aggregates by destabilizing the β-sheet secondary structure under illumination of white light-emitting diode light. We validated that photoexcited [Ru(bpy)3]2+ causes oxidative damages of Aβ peptides, resulting in the dissociation of Aβ aggregates. The efficacy of [Ru(bpy)3]2+ is attributed to reactive oxygen species, such as singlet oxygen, generated from [Ru(bpy)3]2+ that absorbed photon energy in the visible range. Furthermore, photoexcited [Ru(bpy)3]2+ strongly inhibited the self-assembly of Aβ monomers even at concentrations as low as 1 nM and reduced the cytotoxicity of Aβ aggregates.
Statement of Significance
Alzheimer’s disease is the most common progressive neurodegenerative disease, affecting more than 13% of the population over age 65. Over the last decades, researchers have focused on understanding the mechanism of amyloid formation, the hallmark of various amyloid diseases including Alzheimer’s and Parkinson’s. In this paper, we successfully demonstrate the dissociation of β-Amyloid (Aβ) aggregates into small, less-amyloidic fragments by photoexcited [Ru(bpy)3]2+ through destabilization of β-sheet secondary structure. We validated the light-triggered dissociation of amyloid structure using multiple analytical tools. Furthermore, we confirmed that photoexcited [Ru(bpy)3]2+ reduces cytotoxicity of Aβ aggregates. Our work should open a new horizon in the study of Alzheimer's amyloid aggregation by showing the potential of photoexcited dye molecules as an alternative therapeutic strategy for treating Alzheimer’s disease in future.
中文翻译:

钌(II)配合物将自组装的β淀粉状蛋白聚集物光触发解离成小的无毒片段
β-淀粉样蛋白(Aβ)肽自组装成高度稳定的噬菌斑是阿尔茨海默氏病的主要标志。在这里,我们报告了使用钌(II)络合物{[Ru(bpy)3 ] 2+ }的可见光驱动的富含β-折叠的Aβ聚集体分解为小的无毒碎片,该络合物起着高度敏感,生物相容性,光响应性的作用。 -Aβ剂。根据我们使用硫代黄素T,二辛可宁酸,动态光散射,原子力显微镜,圆二色性和傅立叶变换红外光谱法进行的多次分析,[Ru(bpy)3 ] 2+通过破坏β-折叠二级结构的稳定性成功地分解了Aβ聚集体。在白色发光二极管灯的照亮下。我们验证了光激发的[Ru(bpy)3 ] 2+引起Aβ肽的氧化损伤,导致Aβ聚集体解离。[Ru(bpy)3 ] 2+的功效归因于从可见光范围吸收光子能量的[Ru(bpy)3 ] 2+产生的活性氧,例如单线态氧。此外,即使在低至1 nM的浓度下,受光激发的[Ru(bpy)3 ] 2+也会强烈抑制Aβ单体的自组装,并降低Aβ聚集体的细胞毒性。
重要声明
阿尔茨海默氏病是最常见的进行性神经退行性疾病,在65岁以上的人群中影响超过13%的人口。在过去的几十年中,研究人员致力于了解淀粉样蛋白形成的机制,淀粉样蛋白是各种淀粉样疾病的标志,包括阿尔茨海默氏症和帕金森氏症。在本文中,我们成功地证明了β-淀粉样蛋白(Aβ)团簇通过光激发[Ru(bpy)3 ] 2+引起的β-折叠二级结构失稳而解离成小的淀粉样碎片。我们使用多种分析工具验证了淀粉样结构的光触发解离。此外,我们确认了光激发的[Ru(bpy)3 ] 2+降低Aβ聚集体的细胞毒性。我们的工作应通过显示光激发染料分子作为未来治疗阿尔茨海默氏病的替代治疗策略的潜力,为阿尔茨海默氏症淀粉样蛋白聚集的研究开辟新的视野。











































 京公网安备 11010802027423号
京公网安备 11010802027423号