当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Faradaic Spectroscopy
ChemElectroChem ( IF 3.5 ) Pub Date : 2017-11-03 , DOI: 10.1002/celc.201700784 Dijana Jadreško 1 , Dariusz Guziejewski 2 , Valentin Mirčeski 3
ChemElectroChem ( IF 3.5 ) Pub Date : 2017-11-03 , DOI: 10.1002/celc.201700784 Dijana Jadreško 1 , Dariusz Guziejewski 2 , Valentin Mirčeski 3
Affiliation
|
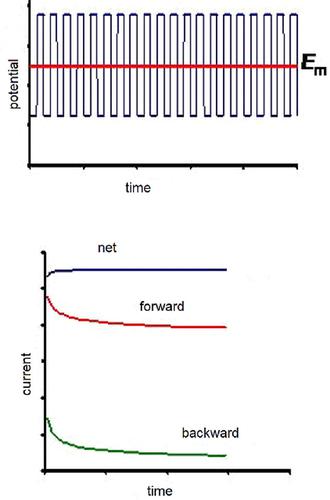
For the purpose of simplification of the electrochemical study under conditions of square‐wave voltammetry (SWV), a simplified form of potential modulation is proposed by replacing the staircase potential ramp with a constant potential. The resulting potential modulation, which could be considered as a pulse form of a chronoamperometric experiment (i. e., square‐wave chronoamperometry), is provisionally termed as electrochemical faradaic spectroscopy, considering the fact that the main tool in the kinetic analysis is the frequency of the potential pulses applied. The proposed technique is illustrated with theoretical analysis of a simple, kinetically controlled electrode reaction of a dissolved redox couple and experimentally verified with the Eu3+(aq)/Eu2+(aq) redox couple at a mercury electrode. The square‐wave chronoamperometry exhibits unique features of the response absent in both conventional SWV and chronoamperometry, widening the range of accessible electrode kinetics.
中文翻译:

电化学法拉第光谱
为了简化方波伏安法(SWV)条件下的电化学研究,提出了一种简化形式的电势调制,方法是用恒定电势代替阶梯电势斜坡。考虑到动力学分析中的主要工具是电化学法拉第谱,由此产生的电势调制可被视为计时安培实验(即方波计时安培)的脉冲形式,暂时称为电化学法拉第谱。施加了潜在的脉冲。通过对溶解的氧化还原对进行简单,动力学控制的电极反应的理论分析来说明所提出的技术,并通过Eu 3+(aq)/ Eu 2+进行了实验验证水银电极上的(aq)氧化还原对。方波计时电流分析法展现了传统SWV和计时电流分析法所缺乏的独特响应特性,拓宽了可触及的电极动力学范围。
更新日期:2017-11-03
中文翻译:

电化学法拉第光谱
为了简化方波伏安法(SWV)条件下的电化学研究,提出了一种简化形式的电势调制,方法是用恒定电势代替阶梯电势斜坡。考虑到动力学分析中的主要工具是电化学法拉第谱,由此产生的电势调制可被视为计时安培实验(即方波计时安培)的脉冲形式,暂时称为电化学法拉第谱。施加了潜在的脉冲。通过对溶解的氧化还原对进行简单,动力学控制的电极反应的理论分析来说明所提出的技术,并通过Eu 3+(aq)/ Eu 2+进行了实验验证水银电极上的(aq)氧化还原对。方波计时电流分析法展现了传统SWV和计时电流分析法所缺乏的独特响应特性,拓宽了可触及的电极动力学范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号