当前位置:
X-MOL 学术
›
Microchem. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparing chemometric and Langmuir isotherm for determination of maximum capacity adsorption of arsenic by a biosorbent
Microchemical Journal ( IF 4.9 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.microc.2017.11.005 J.C. Vieira , L.C. Soares , R.E.S. Froes-Silva
Microchemical Journal ( IF 4.9 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.microc.2017.11.005 J.C. Vieira , L.C. Soares , R.E.S. Froes-Silva
|
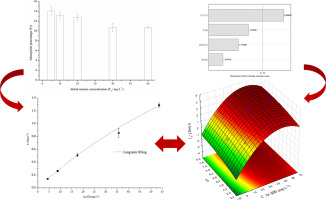
Abstract The determination of the maximum adsorption capacity for a biosorbent is crucial to evaluate the adsorption performance. This parameter is usually obtained from the Langmuir isotherm because it involves very simple and low-cost procedures. Nevertheless, acquisition of adsorption isotherm can be very laborious. Multivariate optimization can be used to improve the determination of the maximum adsorption capacity. In this work, Central Composite Design (CCD) and Surface Response Methodology (SRM) are used for a reliable determination of maximum adsorption capacity of arsenic by a biosorbent of lettuce flour and the result was very close to the value obtained from the Langmuir isotherm.
中文翻译:

比较化学计量学和朗缪尔等温线以确定生物吸附剂对砷的最大吸附能力
摘要 生物吸附剂最大吸附容量的测定对于评价吸附性能至关重要。该参数通常从朗缪尔等温线获得,因为它涉及非常简单和低成本的程序。然而,获得吸附等温线可能非常费力。多变量优化可用于改进最大吸附容量的确定。在这项工作中,中心复合设计 (CCD) 和表面响应方法 (SRM) 被用于可靠测定生菜粉生物吸附剂对砷的最大吸附容量,结果非常接近从朗缪尔等温线获得的值。
更新日期:2018-03-01
中文翻译:

比较化学计量学和朗缪尔等温线以确定生物吸附剂对砷的最大吸附能力
摘要 生物吸附剂最大吸附容量的测定对于评价吸附性能至关重要。该参数通常从朗缪尔等温线获得,因为它涉及非常简单和低成本的程序。然而,获得吸附等温线可能非常费力。多变量优化可用于改进最大吸附容量的确定。在这项工作中,中心复合设计 (CCD) 和表面响应方法 (SRM) 被用于可靠测定生菜粉生物吸附剂对砷的最大吸附容量,结果非常接近从朗缪尔等温线获得的值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号