Bioelectrochemistry ( IF 4.8 ) Pub Date : 2017-11-11 , DOI: 10.1016/j.bioelechem.2017.11.004 Marina Mlakar , Vlado Cuculić , Sanja Frka , Blaženka Gašparović
|
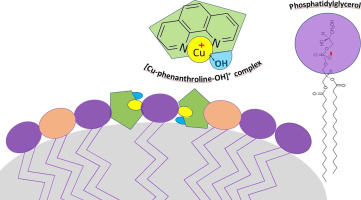
Detailed investigation of Cu (II) binding with natural lipid phosphatidylglycerol (PG) in aqueous solution was carried out by voltammetric measurements at the mercury drop electrode, complemented by monolayer studies in a Langmuir trough and electrophoretic measurements, all used as models for hydrophobic cell membranes. Penetration of copper ions into the PG layer was facilitated by the formation of hydrophilic Cu-Phenanthroline (Phen) complex in the subphase, followed by the mixed ligand Cu-Phen-PG complex formation at the hydrophobic interface. Electrophoretic measurements indicated a comparatively low abundance of the formed mixed ligand complex within the PG vesicles, resulting it the zeta potential change of + 0.83 mV, while monolayer studies confirmed their co-existence at the interface. The Cu-Phen-PG complex was identified in the pH range from 6 to 9. The stoichiometry of the complex ([PhenCuOHPG]), as well as its stability and kinetics of formation, were determined at the mercury drop electrode. Cu-Phen-PG reduces quasireversibly at about − 0.7 V vs. Ag/AgCl including reactant adsorption, followed by irreversible mixed complex dissociation, indicating a two-electron transfer - chemical reaction (EC mechanism). Consequently, the surface concentration (γ) of the adsorbed [PhenCuOHPG] complex at the hydrophobic electrode surface was calculated to be (3.35 ± 0.67) × 10− 11 mol cm− 2. Information on the mechanism of Cu (II) - lipid complex formation is a significant contribution to the understanding of complex processes at natural cell membranes.
中文翻译:

细胞膜上的铜-磷脂相互作用模拟疏水表面
在汞滴电极上通过伏安法测量,对铜(II)与天然脂质磷脂酰甘油(PG)的结合进行了详细的研究,辅之以在朗缪尔(Langmuir)槽中的单层研究和电泳测量,所有这些都用作疏水性细胞膜的模型。通过在亚相中形成亲水性铜-邻菲咯啉(Phen)配合物,然后在疏水界面形成混合配体Cu-Phen-PG配合物,可促进铜离子渗透到PG层中。电泳测量表明,PG囊泡中形成的混合配体复合物相对较低的丰度,导致zeta电位变化为+ 0.83 mV,而单层研究证实了它们在界面处共存。在6至9的pH范围内鉴定出Cu-Phen-PG配合物。在汞滴电极上测定了配合物的化学计量([PhenCuOHPG])及其稳定性和形成动力学。Cu-Phen-PG在约− 0.7 V时相对于Ag / AgCl发生近似还原,包括反应物吸附,随后发生不可逆的混合络合物解离,表明发生了两电子转移-化学反应(EC机理)。因此,表面浓度(疏水电极表面吸附的[PhenCuOHPG]复合物的γ)经计算为(3.35±0.67)×10 -11 mol cm -2。Cu(II)-脂质复合物形成机理的信息对理解天然细胞膜的复杂过程有重要贡献。









































 京公网安备 11010802027423号
京公网安备 11010802027423号