Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.jcou.2017.11.009 Irene Ham-Liu , J. Arturo Mendoza-Nieto , Heriberto Pfeiffer
|
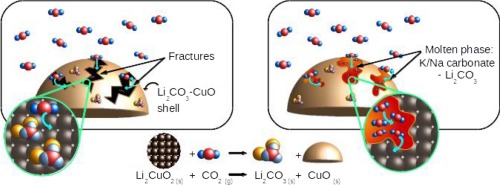
This work presents a proposal for enhancing CO2 capture over lithium cuprate (Li2CuO2) by alkaline carbonates addition. Lithium cuprate was synthesized via solid-state reaction. Subsequently, portions of Li2CuO2 were mechanically mixed with 10 wt% of potassium carbonate (K2CO3), sodium carbonate (Na2CO3) or equal amounts of both carbonates (5–5 wt%). All samples were characterized by several techniques and then CO2 chemisorption process was evaluated on different dynamic and isothermal conditions. The presence of K and/or Na carbonates preserves the primary properties of pristine Li2CuO2, such as crystalline phase (DRX) and microstructure (SEM), also allowing to increase textural properties (N2 physisorption) and modifying CO2 desorption abilities (TPD), in comparison with pure material. In general, carbonates addition induces some changes during CO2 chemisorption, depending on the type of carbonate used. On the isothermal tests, it was observed that between 400 and 600 °C the sample containing both carbonates presented the best capture performance, capturing between 25.7 and 31.8 wt% of CO2 (63.9–79.1% of efficiency). On the other hand, at 700 °C, Na-Li2CuO2 sample presented the best capture ability, capturing 30.6 wt% of CO2 (76.1% of efficiency). Meanwhile at 650 °C, K-Li2CuO2 presented the highest sorption capacity with 40.4 wt% of CO2 captured, which represents ∼100% of efficiency. The above results showed that in the same thermal conditions, samples modified with alkaline carbonates improved the CO2 capture process. This enhancement was attributed to the formation of eutectic phases (observed in DSC analysis) between sodium and potassium carbonates added mechanically and lithium carbonate (Li2CO3) formed as result of CO2 chemisorption process. Finally, it was observed that carbonate addition is a feasible way to increase CO2 capture in Li2CuO2 material by means of eutectic phase formation.
中文翻译:

在Li 2 CuO 2上添加K 2 CO 3-和Na 2 CO 3-产生的CO 2化学吸附增强作用
这项工作提出了通过添加碱性碳酸盐来增强铜酸锂(Li 2 CuO 2)上的CO 2捕集的建议。通过固相反应合成铜酸锂。随后,将部分Li 2 CuO 2与10 wt%的碳酸钾(K 2 CO 3),碳酸钠(Na 2 CO 3)或等量的两种碳酸盐(5-5 wt%)机械混合。所有样品均通过多种技术进行表征,然后再进行CO 2表征。在不同的动态和等温条件下评估化学吸附过程。碳酸钾和/或碳酸钠的存在保留了原始的Li 2 CuO 2的主要性质,例如晶相(DRX)和微观结构(SEM),还可以提高织构性质(N 2物理吸附)并改变CO 2的解吸能力(TPD),与纯材料相比。通常,根据所用碳酸盐的类型,碳酸盐的添加会在CO 2化学吸附过程中引起一些变化。在等温测试中,观察到在400至600°C的温度下,含有两种碳酸盐的样品表现出最佳的捕获性能,捕获25.7至31.8 wt%的CO 2(效率的63.9–79.1%)。另一方面,在700°C下,Na-Li 2 CuO 2样品表现出最佳的捕获能力,捕获了30.6 wt%的CO 2(效率的76.1%)。同时,在650°C时,K-Li 2 CuO 2表现出最高的吸附容量,捕获到40.4 wt%的CO 2,约占效率的100%。以上结果表明,在相同的热条件下,用碱性碳酸盐改性的样品可以改善CO 2的捕集过程。这种增强归因于在机械添加的碳酸钠和碳酸钾与碳酸锂(Li 2 CO 3)之间形成了共晶相(在DSC分析中观察到)。)是由于CO 2化学吸附过程而形成的。最后,观察到添加碳酸盐是通过共晶相形成来增加Li 2 CuO 2材料中CO 2捕获的可行方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号