Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2017-10-09 , DOI: 10.1016/j.jcou.2017.09.006 Manuri Brahmayya , Shenghong A. Dai , Shing-Yi Suen
|
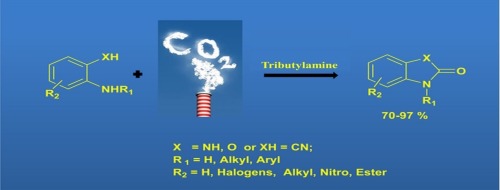
An efficient synthesis for the benzimidazolones and various 1,3-disubstituted urea derivatives prepared from several nucleophiles like o-phenylenediamines and o-aminophenols with CO2 (1 MPa) under transition-metal-free or acetate complex free conditions has been developed. A variety of chemicals were synthesized in moderate to good yields promoted by tributylamine (TBA) using the nucleophiles and CO2 as a green and renewable C1 source. The cyclization of CO2 and o-phenylenediamine offered a general and straightforward synthesis of benzimidazolones and cyclic ureas promoted by the inexpensive and environmentally-friendly TBA as the carbonylation catalyst. Thus, various valuable cyclic ureas and their carbonyl homologs were synthesized in good yields through this green carbonylation process.
中文翻译:

通过邻苯二胺与CO 2羰基化轻松合成2-苯并咪唑酮
已开发了在无过渡金属或无乙酸盐配合物的条件下,由几种亲核试剂如邻苯二胺和邻氨基苯酚与CO 2(1 MPa)制备的苯并咪唑酮和各种1,3-二取代脲衍生物的有效合成方法。使用亲核试剂和CO 2作为绿色和可再生的C1来源,通过三丁胺(TBA)可以中等至良好的产率合成多种化学物质。CO 2和o的环化苯二胺可通过廉价和环保的TBA作为羰基化催化剂促进苯并咪唑酮和环状脲的一般直接合成。因此,通过这种绿色羰基化过程以高收率合成了各种有价值的环状脲及其羰基同系物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号