当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
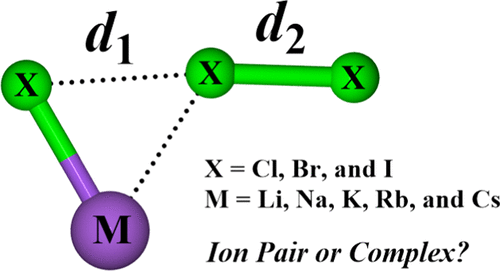
Alkali-Metal Trihalides: M+X3– Ion Pair or MX–X2 Complex?
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1021/acs.jpcb.7b10005 Zhi Sun 1 , Kevin B. Moore 1 , J. Grant Hill 2 , Kirk A. Peterson 3 , Henry F. Schaefer 1 , Roald Hoffmann 4
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1021/acs.jpcb.7b10005 Zhi Sun 1 , Kevin B. Moore 1 , J. Grant Hill 2 , Kirk A. Peterson 3 , Henry F. Schaefer 1 , Roald Hoffmann 4
Affiliation
|
The alkali-metal trihalides MX3 (M = Li, Na, K, Rb, and Cs; X = Cl, Br, and I) are systematically studied using coupled-cluster methods. Benchmarks using CCSD(T) against diatomic experimental results suggest satisfactory performance for the weighted core-valence basis sets (new basis sets for K, Rb, and Cs) selected for predicting reliable structures and harmonic vibrational frequencies. An isomer search using the B3LYP functional yields a planar, yet asymmetric T-shaped Cs structure as the global minimum for all MX3 species. Much higher level CCSD(T) computations show a moderate to strong distortion of the X3– anion by the M+ cation in the respective equilibrium geometries. Most obviously, for LiCl3, the two Cl–Cl distances are separated by 0.786 Å. Even for CsI3, the structure least distorted from the M+X3– model, the two I–I distances differ by 0.243 Å. It does not take much energy to distort the parent anions along an antisymmetric stretch, so this is no surprise. The normal modes of vibration of the MX3 molecules are in better agreement with matrix isolation experiments than previous calculations. And these normal modes reveal that, instead of the well-established antisymmetric and symmetric stretches of the “free” X3– anions, relatively localized and mutually perturbed X–X and M–X stretches are calculated. The suggestion emerges that the MX3 system may be alternatively described as an MX–X2 complex rather than the M+X3– ion pair. This perspective is supported by bonding analyses showing low electron densities at the bond critical points and natural bond orders between the MX and X2 moieties. The thermochemistry of fragmentations of MX3 to MX + X2 versus M+ + X3– also supports the alternative viewpoint of the bonding in this class of molecules.
中文翻译:

碱金属三卤化物:M + X 3 –离子对或MX–X 2复合物?
使用耦合簇方法系统地研究了碱金属三卤化物MX 3(M = Li,Na,K,Rb和Cs; X = Cl,Br和I)。针对双原子实验结果使用CCSD(T)进行的基准测试表明,为预测可靠的结构和谐波振动频率而选择的加权核心价基集(K,Rb和Cs的新基集)具有令人满意的性能。使用B3LYP官能团进行异构体搜索会产生一个平面但不对称的T形C s结构,作为所有MX 3物种的整体最小值。高得多的电平CCSD(T)计算表明中度到X的强失真3 -阴离子由M +各自平衡几何中的阳离子。最明显的是,对于LiCl 3,两个Cl–Cl的距离相隔0.786Å。即使对于Ms + X 3 –模型失真最小的CsI 3,两个I–I距离也相差0.243Å。沿反对称拉伸使母体阴离子变形不需要太多的能量,因此这不足为奇。与以前的计算相比,MX 3分子的正常振动模式与基质分离实验更加吻合。这些正常模式揭示了,而不是公认的“自由” X 3的反对称和对称拉伸–计算相对局部且相互干扰的X–X和M–X拉伸的阴离子。提出的建议是,可以将MX 3系统描述为MX–X 2络合物,而不是M + X 3 –离子对。这种观点得到了键合分析的支持,键合分析显示了在键的临界点处的电子密度低以及MX和X 2部分之间的自然键序。MX的碎裂的热化学3至MX + X 2相对于中号+ + X 3 -还支持接合的在这个类分子的替代性观点。
更新日期:2017-12-12
中文翻译:

碱金属三卤化物:M + X 3 –离子对或MX–X 2复合物?
使用耦合簇方法系统地研究了碱金属三卤化物MX 3(M = Li,Na,K,Rb和Cs; X = Cl,Br和I)。针对双原子实验结果使用CCSD(T)进行的基准测试表明,为预测可靠的结构和谐波振动频率而选择的加权核心价基集(K,Rb和Cs的新基集)具有令人满意的性能。使用B3LYP官能团进行异构体搜索会产生一个平面但不对称的T形C s结构,作为所有MX 3物种的整体最小值。高得多的电平CCSD(T)计算表明中度到X的强失真3 -阴离子由M +各自平衡几何中的阳离子。最明显的是,对于LiCl 3,两个Cl–Cl的距离相隔0.786Å。即使对于Ms + X 3 –模型失真最小的CsI 3,两个I–I距离也相差0.243Å。沿反对称拉伸使母体阴离子变形不需要太多的能量,因此这不足为奇。与以前的计算相比,MX 3分子的正常振动模式与基质分离实验更加吻合。这些正常模式揭示了,而不是公认的“自由” X 3的反对称和对称拉伸–计算相对局部且相互干扰的X–X和M–X拉伸的阴离子。提出的建议是,可以将MX 3系统描述为MX–X 2络合物,而不是M + X 3 –离子对。这种观点得到了键合分析的支持,键合分析显示了在键的临界点处的电子密度低以及MX和X 2部分之间的自然键序。MX的碎裂的热化学3至MX + X 2相对于中号+ + X 3 -还支持接合的在这个类分子的替代性观点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号