当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simulation of Calcium Phosphate Species in Aqueous Solution: Force Field Derivation
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jpcb.7b10697 Raffaella Demichelis 1 , Natalya A. Garcia 1 , Paolo Raiteri 1 , Riccardo Innocenti Malini 2, 3 , Colin L. Freeman 2 , John H. Harding 2 , Julian D. Gale 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jpcb.7b10697 Raffaella Demichelis 1 , Natalya A. Garcia 1 , Paolo Raiteri 1 , Riccardo Innocenti Malini 2, 3 , Colin L. Freeman 2 , John H. Harding 2 , Julian D. Gale 1
Affiliation
|
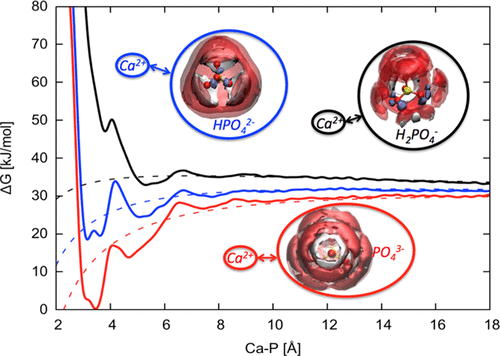
A new force field has been derived for the aqueous calcium phosphate system that aims to reproduce the key thermodynamic properties of the system, including free energies of hydration of the ions and the solubility of the solid mineral phases. Interactions of three phosphate anions (PO43–, HPO42–, and H2PO4–) with water were calibrated through comparison with the results obtained from ab initio molecular dynamics using both GGA and hybrid density functional theory with dispersion corrections. In the solid state, the force field has been evaluated by benchmarking against experiment and other existing models and is shown to reproduce the structural and mechanical properties well, despite the primary focus being on thermodynamics. To validate the force field, the thermodynamics of ion pairing for calcium phosphate species in water has been computed and shown to be in excellent agreement with experimental data.
中文翻译:

水溶液中磷酸钙种类的模拟:力场推导
已经为水性磷酸钙系统获得了一个新的力场,该力场旨在重现该系统的关键热力学性质,包括离子水合的自由能和固体矿物相的溶解度。三种磷酸根阴离子(PO 4 3–,HPO 4 2–和H 2 PO 4 –的相互作用通过使用GGA和混合密度泛函理论以及色散校正,通过与从头算分子动力学获得的结果进行比较,对水进行了校准。在固态下,力场已通过以实验和其他现有模型为基准进行了评估,并且显示出很好地再现了结构和机械性能,尽管主要侧重于热力学。为了验证力场,已经计算了水中磷酸钙物质的离子对的热力学,并且与实验数据非常吻合。
更新日期:2018-01-19
中文翻译:

水溶液中磷酸钙种类的模拟:力场推导
已经为水性磷酸钙系统获得了一个新的力场,该力场旨在重现该系统的关键热力学性质,包括离子水合的自由能和固体矿物相的溶解度。三种磷酸根阴离子(PO 4 3–,HPO 4 2–和H 2 PO 4 –的相互作用通过使用GGA和混合密度泛函理论以及色散校正,通过与从头算分子动力学获得的结果进行比较,对水进行了校准。在固态下,力场已通过以实验和其他现有模型为基准进行了评估,并且显示出很好地再现了结构和机械性能,尽管主要侧重于热力学。为了验证力场,已经计算了水中磷酸钙物质的离子对的热力学,并且与实验数据非常吻合。









































 京公网安备 11010802027423号
京公网安备 11010802027423号