当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water’s Thermal Pressure Drives the Temperature Dependence of Hydrophobic Hydration
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b11100 Claudio A. Cerdeiriña 1 , Pablo G. Debenedetti 2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b11100 Claudio A. Cerdeiriña 1 , Pablo G. Debenedetti 2
Affiliation
|
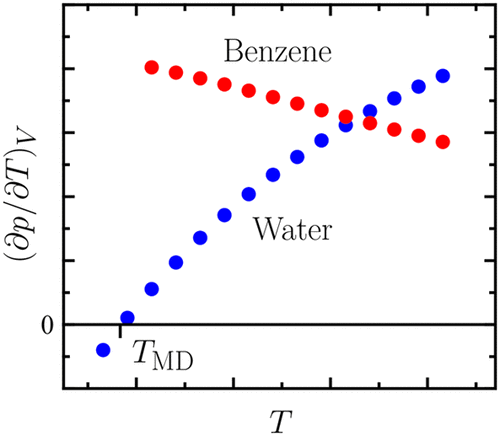
With the aid of literature experimental data and reported results from molecular simulation, two thermodynamic relations are found to provide a theoretical basis for the understanding of a variety of characteristic features associated with the solvation of small nonpolar molecules in water. Thus, the large and positive solvation heat capacity, enthalpy–entropy compensation, the solubility minimum and solvation free energy maximum with respect to temperature, enthalpy convergence, and entropy convergence are rationalized in a unified way. Our key finding is that all of these phenomena are driven by the thermal pressure coefficient of pure water, which, via the isobaric thermal expansivity and the isothermal compressibility, reflects its unusual thermodynamics. Remarkably, the solubility minimum is found to be a direct consequence of water’s density maximum.
中文翻译:

水的热压驱动疏水水化的温度依赖性
借助文献实验数据和分子模拟报告的结果,发现了两个热力学关系,为理解与非极性小分子在水中的溶解相关的各种特征提供了理论基础。因此,以统一的方式合理化了相对于温度而言大而正的溶剂化热容量,焓-熵补偿,溶解度最小值和溶剂化自由能最大值(相对于温度),焓收敛和熵收敛。我们的关键发现是所有这些现象都是由纯水的热压系数驱动的,纯水的热压系数通过等压热膨胀系数和等温可压缩性反映了其非同寻常的热力学。值得注意的是
更新日期:2017-12-11
中文翻译:

水的热压驱动疏水水化的温度依赖性
借助文献实验数据和分子模拟报告的结果,发现了两个热力学关系,为理解与非极性小分子在水中的溶解相关的各种特征提供了理论基础。因此,以统一的方式合理化了相对于温度而言大而正的溶剂化热容量,焓-熵补偿,溶解度最小值和溶剂化自由能最大值(相对于温度),焓收敛和熵收敛。我们的关键发现是所有这些现象都是由纯水的热压系数驱动的,纯水的热压系数通过等压热膨胀系数和等温可压缩性反映了其非同寻常的热力学。值得注意的是



























 京公网安备 11010802027423号
京公网安备 11010802027423号