当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
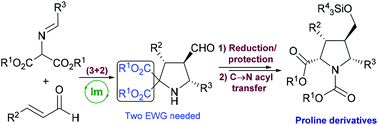
Highly diastereoselective C → N acyl rearrangement in polysubstituted pyrrolidine 2,2-dicarboxylates. Stereocontrolled synthesis of densely functionalized prolines†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7qo00793k Silvia Reboredo 1, 2, 3, 4 , Ainara García-Marijuan 1, 2, 3, 4 , Uxue Uria 1, 2, 3, 4 , Efraím Reyes 1, 2, 3, 4 , Luisa Carrillo 1, 2, 3, 4 , Iratxe Ugarriza 1, 2, 3, 4 , Jose L. Vicario 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7qo00793k Silvia Reboredo 1, 2, 3, 4 , Ainara García-Marijuan 1, 2, 3, 4 , Uxue Uria 1, 2, 3, 4 , Efraím Reyes 1, 2, 3, 4 , Luisa Carrillo 1, 2, 3, 4 , Iratxe Ugarriza 1, 2, 3, 4 , Jose L. Vicario 1, 2, 3, 4
Affiliation
|
An efficient and easy protocol for promoting a C → N acyl transfer reaction in diverse enantiopure densely substituted pyrrolidine 2,2-dicarboxylates was performed, yielding the corresponding proline ester derivatives with yields in the range of 68–99% and total diastereoselection (dr > 95 : 5 in all cases). The substitution patterns both at C-3 and C-5 present in starting pyrrolidines as well as the viability of presenting different alkoxycarbonyl moieties have been evaluated observing good reaction performance in all cases.
中文翻译:

在多取代的吡咯烷2,2-二羧酸酯中具有非对映选择性的C→N酰基重排。立体控制的稠密官能化脯氨酸的合成†
进行了有效而简便的方案,以促进各种对映纯的稠密取代的吡咯烷2,2-二羧酸酯中的C→N酰基转移反应,得到相应的脯氨酸酯衍生物,产率在68–99%范围内,总非对映选择性(dr> 95:5)。在所有情况下均观察到良好的反应性能,已评估了起始吡咯烷中存在的C-3和C-5处的取代方式以及呈现不同烷氧基羰基部分的可行性。
更新日期:2017-12-12
中文翻译:

在多取代的吡咯烷2,2-二羧酸酯中具有非对映选择性的C→N酰基重排。立体控制的稠密官能化脯氨酸的合成†
进行了有效而简便的方案,以促进各种对映纯的稠密取代的吡咯烷2,2-二羧酸酯中的C→N酰基转移反应,得到相应的脯氨酸酯衍生物,产率在68–99%范围内,总非对映选择性(dr> 95:5)。在所有情况下均观察到良好的反应性能,已评估了起始吡咯烷中存在的C-3和C-5处的取代方式以及呈现不同烷氧基羰基部分的可行性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号