当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Convenient synthesis of 2-amino-3-(arylthio)indoles via the Rh-catalyzed reaction of 3-diazoindol-2-imines with thioesters†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7ob02597a Fanghui Ma 1, 2, 3, 4 , Jing Qian 1, 2, 3, 4 , Ping Lu 1, 2, 3, 4 , Yanguang Wang 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7ob02597a Fanghui Ma 1, 2, 3, 4 , Jing Qian 1, 2, 3, 4 , Ping Lu 1, 2, 3, 4 , Yanguang Wang 1, 2, 3, 4
Affiliation
|
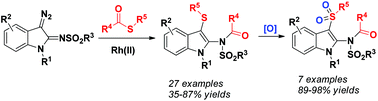
2-Amino-3-(arylthio)indoles were conveniently synthesized via the Rh(II)-catalyzed C–S/N–C coupling reaction between 3-diazoindol-2-imines and thioesters. The products could be further oxidized to 2-amino-3-(arylsulfonyl)indoles by m-chloroperbenzoic acid and the N-sulfonyl group could be easily removed by reduction with SmI2.
中文翻译:

方便的合成2-氨基-3-(芳基硫基)吲哚经由3- diazoindol -2-亚胺与硫酯上的Rh催化的反应†
通过Rh(II)催化的3-重氮吲哚-2-亚胺和硫代酸酯之间的C-S / N-C偶联反应可以方便地合成2-氨基-3-(芳硫基)吲哚。该产品可以被进一步氧化成2-氨基-3-(芳基磺酰基)吲哚由米氯过苯甲酸和Ñ磺酰基团可通过还原SMI容易地除去2。
更新日期:2017-12-12
中文翻译:

方便的合成2-氨基-3-(芳基硫基)吲哚经由3- diazoindol -2-亚胺与硫酯上的Rh催化的反应†
通过Rh(II)催化的3-重氮吲哚-2-亚胺和硫代酸酯之间的C-S / N-C偶联反应可以方便地合成2-氨基-3-(芳硫基)吲哚。该产品可以被进一步氧化成2-氨基-3-(芳基磺酰基)吲哚由米氯过苯甲酸和Ñ磺酰基团可通过还原SMI容易地除去2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号