当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A specific demetalation of Fe–N4 catalytic sites in the micropores of NC_Ar + NH3 is at the origin of the initial activity loss of the highly active Fe/N/C catalyst used for the reduction of oxygen in PEM fuel cells†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7ee02302b Régis Chenitz 1, 2, 3, 4 , Ulrike I. Kramm 5, 6, 7, 8, 9 , Michel Lefèvre 3, 4, 10 , Vassili Glibin 4, 11, 12, 13 , Gaixia Zhang 1, 2, 3, 4 , Shuhui Sun 1, 2, 3, 4 , Jean-Pol Dodelet 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7ee02302b Régis Chenitz 1, 2, 3, 4 , Ulrike I. Kramm 5, 6, 7, 8, 9 , Michel Lefèvre 3, 4, 10 , Vassili Glibin 4, 11, 12, 13 , Gaixia Zhang 1, 2, 3, 4 , Shuhui Sun 1, 2, 3, 4 , Jean-Pol Dodelet 1, 2, 3, 4
Affiliation
|
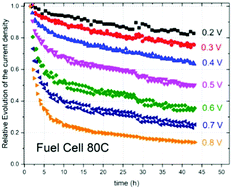
In this study, we explored the behavior of NC_Ar + NH3, an initially highly active catalyst for oxygen electroreduction, in H2/air fuel cells from 0.8 to 0.2 V at 80 °C and 25 °C, in order to find the causes of its instability. We discovered that the decay of the current density always involves the superposition of fast and slow first order kinetics, for which half-lives were obtained. The half-life of the fast decay was practically the same at all potentials and temperatures with a value of around 138 ± 55 min, while the half-life of the slow decay greatly varied from a minimum of ≈2400 min (40 h) to infinity. From the adsorption–desorption isotherm of NC_Ar + NH3, it was deduced that the Fe/N/C carbonaceous catalyst is characterized by interconnected open-end slit-shaped micropores, in which water (with dissolved H+ and O2) quickly flows in the fuel cells if their width is ≥0.7 nm as it has no interaction with the hydrophobic walls of the micropores. The driving force of this quick water flow is the humidified air streaming through the working cathode. Fe–N4-like active sites are thermodynamically stable in stagnant acidic conditions, but according to the Le Chatelier principle, they demetalate in the flux of water running into the micropores. This specific demetalation is the cause of the initial loss of ORR activity of NC_Ar + NH3 catalysts assigned to the fast current decay in fuel cells.
中文翻译:

在NC_Ar + NH 3的微孔中 ,Fe–N 4催化位点的特定脱金属是用于降低PEM燃料电池中氧的高活性Fe / N / C催化剂的初始活性损失的起点†
在这项研究中,我们探索NC_Ar + NH的行为3,用于氧气电还原初始高活性的催化剂,H中2 /空气燃料电池在0.8至0.2V的在80℃和25℃下,为了找到原因其不稳定性。我们发现电流密度的衰减总是涉及快和慢一级动力学的叠加,为此获得了半衰期。快速衰减的半衰期在所有电势和温度下几乎都相同,其值约为138±55分钟,而缓慢衰减的半衰期则从最小≈2400分钟(40 h)到最小。无穷。从NC_Ar + NH 3的吸附-解吸等温线因此,推论出Fe / N / C碳质催化剂的特征是具有相互连接的开口狭缝形微孔,当宽度≥0.7nm时,水(溶解有H +和O 2)会迅速在燃料电池中流动。因为它与微孔的疏水壁没有相互作用。这种快速水流的驱动力是流过工作阴极的加湿空气。类似于Fe–N 4的活性位点在停滞的酸性条件下是热力学稳定的,但是根据Le Chatelier原理,它们在流入微孔的水流中会脱金属。这种特定的脱金属是分配给燃料电池中快速电流衰减的NC_Ar + NH 3催化剂的ORR活性最初损失的原因。
更新日期:2017-12-12
中文翻译:

在NC_Ar + NH 3的微孔中 ,Fe–N 4催化位点的特定脱金属是用于降低PEM燃料电池中氧的高活性Fe / N / C催化剂的初始活性损失的起点†
在这项研究中,我们探索NC_Ar + NH的行为3,用于氧气电还原初始高活性的催化剂,H中2 /空气燃料电池在0.8至0.2V的在80℃和25℃下,为了找到原因其不稳定性。我们发现电流密度的衰减总是涉及快和慢一级动力学的叠加,为此获得了半衰期。快速衰减的半衰期在所有电势和温度下几乎都相同,其值约为138±55分钟,而缓慢衰减的半衰期则从最小≈2400分钟(40 h)到最小。无穷。从NC_Ar + NH 3的吸附-解吸等温线因此,推论出Fe / N / C碳质催化剂的特征是具有相互连接的开口狭缝形微孔,当宽度≥0.7nm时,水(溶解有H +和O 2)会迅速在燃料电池中流动。因为它与微孔的疏水壁没有相互作用。这种快速水流的驱动力是流过工作阴极的加湿空气。类似于Fe–N 4的活性位点在停滞的酸性条件下是热力学稳定的,但是根据Le Chatelier原理,它们在流入微孔的水流中会脱金属。这种特定的脱金属是分配给燃料电池中快速电流衰减的NC_Ar + NH 3催化剂的ORR活性最初损失的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号