Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-12-11 , DOI: 10.1016/j.jfluchem.2017.12.001 O.V. Andrrev , I.A. Razumkova , A.N. Boiko
|
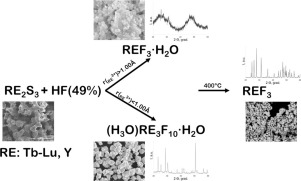
The nature of the interaction between sulphides of the rare-earth elements (RE) of the yttrium subgroup RE2S3 (Dy − Lu, Y) with hydrofluoric acid (concentration of 49%) was studied. Powders of RE2S3 compounds were obtained by the reaction between RE2O3 (Dy − Lu, Y) and Tb4O7 with a stream of H2S and CS2 in the 1000–1050 °C temperature range. The reaction between RE2S3 and HF was carried out in a glassy carbon crucible in the 21–25 °C temperature range in solution, and the precipitate was washed with H2O and dried at 90–100 °C. For the hydrates of RE fluorides with decreasing RE radius, the RE content increases the sorption of water for TbF3·0.5H2O; DyF3·0.5H2O; YF3·0.5H2O; HoF3·0.8H2O; and ErF3·0.9H2O. The powders of REF3·nH2O consisted of micro- and nano-sized particles containing the amorphous phase. Water loss occurs in the 50–180 °C temperature range and is accompanied by a natural increase in enthalpy: from 23.6 J/g for TbF3 · 0.5H2O to 97.8 J/g for ErF3·0.9H2O and from 51.1 J/g for (H3O)Tm3F10·1.7H2O to 64.5 J/g for (H3O)Lu3F10·0.9H2O. Exothermic effects of formation of polycrystalline phase REF3 were recorded in the 200–300 °C temperature range. The reaction of RE2S3 (Tm, Yb, Lu) powders with HF proceeds with the formation of the zeolite type compounds (H3O)Tm3F10·1.7H2O, (H3O)Yb3F10·1.0H2O and (H3O)Lu3F10·0.9H2O. Thermal dissociation occurs with gradual loss of water and HF to form RE fluorides with the β-YF3 structure as described by
中文翻译:

从硫化物前体获得的稀土化合物REF 3,REF 3 · n H 2 O和(H 3 O)RE 3 F 10 · n H 2 O(RE = Tb-Lu,Y)的合成和热稳定性
研究了钇亚组RE 2 S 3(Dy-Lu,Y)的稀土元素(RE)的硫化物与氢氟酸(浓度为49%)之间相互作用的性质。RE 2 S 3化合物的粉末是通过RE 2 O 3(Dy-Lu,Y)和Tb 4 O 7与H 2 S和CS 2的物流在1000-1050°C的温度下反应而获得的。RE 2 S 3和HF之间的反应是在玻璃碳坩埚中于21–25°C的温度范围内在溶液中进行的,沉淀物用H 2洗涤O,并在90–100°C下干燥。对于RE半径减小的RE氟化物的水合物,RE含量增加了TbF 3 · 0.5 H 2 O的水吸附;反之,DyF 3 · 0.5 H 2 O;YF 3 · 0.5 H ^ 2 O; HoF 3 · 0.8 H 2 O;和ERF 3 · 0.9 H ^ 2 REF的O.粉末3 · Ñ ħ 2O由包含非晶相的微米和纳米尺寸的颗粒组成。发生在50-180℃的温度范围内的水损失并且伴随着在焓自然升高:从23.6焦耳/克为TbF系3 · 0.5 H ^ 2 O操作97.8焦耳/克为ERF 3 · 0.9 H ^ 2 O和从51.1焦耳/克为(H 3 O)的Tm 3 ˚F 10 · 1.7 ħ 2 O操作64.5焦耳/克为(H 3 O)路3 ˚F 10 · 0.9 ħ 2形成的多晶相REF的O.放热效果3在200–300°C的温度范围内记录。RE 2 S 3(Tm,Yb,Lu)粉末与HF的反应进行,形成了沸石型化合物(H 3 O)Tm 3 F 10 · 1.7 H 2 O,(H 3 O)Yb 3 F 10 · 1.0 ħ 2 O和(H 3 O)路3 ˚F 10 · 0.9 ħ 2与水和HF逐渐损失到形式RE氟化物与β-YF发生O.热离解3如由结构











































 京公网安备 11010802027423号
京公网安备 11010802027423号