当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Trends in Monoboronyl Compounds: Analysis of the Interaction of Second-Row Elements with BO
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.jpca.7b10482 Pilar Redondo 1 , Víctor M. Rayón 1 , Carmen Barrientos 1 , Antonio Largo 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.jpca.7b10482 Pilar Redondo 1 , Víctor M. Rayón 1 , Carmen Barrientos 1 , Antonio Largo 1
Affiliation
|
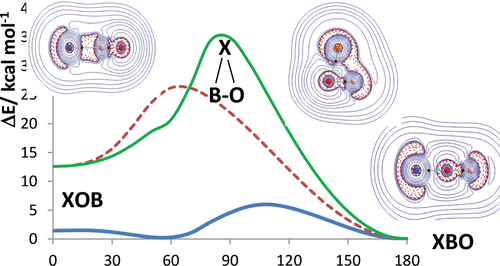
A theoretical study of the monoboronyl compounds of second-row elements, [XBO] (X = Na, Si, P, S, Cl), has been carried out. It is observed that the preference for the XBO arrangement is higher when moving to the right of the period. In the case of sodium monoboronyl three minima were characterized, all lying rather close in energy: linear NaBO, linear NaOB, and an L-shaped structure. Linear NaBO and the L-shaped structure are nearly isoenergetic, whereas linear NaOB is located 2.11 kcal/mol above linear NaBO. The barrier for the conversion of the L-shaped structure into linear NaBO is about 5.1 kcal/mol, suggesting that both species could be potential targets for experimental detection. For silicon monoboronyl, two minima, linear SiBO and linear SiOB, are found, the latter lying about 13 kcal/mol above SiBO. The barrier for the isomerization of SiOB into SiBO is estimated to be 11.4 kcal/mol. For phosphorus, sulfur, and chlorine monoboronyls the linear XBO isomer is clearly the most stable one, and the barriers for the conversion into XOB species are relatively high, suggesting that quite likely the linear XBO isomer should be the main experimental target. All studied monoboronyls are relatively stable, with dissociation energies increasing from left to right of the second-row (69.8 kcal/mol for NaBO and 118.98 kcal/mol for ClBO). An analysis of the bonding for second-row monoboronyls has been carried out, emphasizing the different characteristics of the X–B and X–O bonds along the second row.
中文翻译:

单硼烷基化合物的结构趋势:第二行元素与BO相互作用的分析
对第二行元素的单硼烷基化合物[XBO](X = Na,Si,P,S,Cl)进行了理论研究。可以观察到,当移动到周期的右侧时,对XBO设置的偏好更高。在单硼酸钠钠中,三个极小值的特征都非常接近:线性NaBO,线性NaOB和L形结构。线性NaBO和L形结构几乎是等能量的,而线性NaOB位于线性NaBO之上2.11 kcal / mol。L形结构转化为线性NaBO的势垒约为5.1 kcal / mol,表明这两个物种都可能成为实验检测的潜在目标。对于单硼硅硅,发现了两个最小值,线性SiBO和线性SiOB,后者比SiBO高约13 kcal / mol。SiOB异构化为SiBO的势垒估计为11.4 kcal / mol。对于磷,硫和氯单硼烷基而言,线性XBO异构体显然是最稳定的异构体,而且转化为XOB物种的障碍也相对较高,这表明线性XBO异构体很可能应该是主要的实验目标。所有研究的单硼烷醇都相对稳定,解离能从第二行的左向右增加(NaBO为69.8 kcal / mol,ClBO为118.98 kcal / mol)。已经对第二行单硼烷基的键合进行了分析,强调了第二行X–B和X–O键的不同特征。并且转化为XOB物种的障碍相对较高,这表明线性XBO异构体很可能应该是主要的实验目标。所有研究的单硼烷醇都相对稳定,解离能从第二行的左向右增加(NaBO为69.8 kcal / mol,ClBO为118.98 kcal / mol)。已经对第二行单硼烷基的键合进行了分析,强调了第二行X–B和X–O键的不同特征。并且转化为XOB物种的障碍相对较高,这表明线性XBO异构体很可能应该是主要的实验目标。所有研究的单硼烷醇都相对稳定,解离能从第二行的左向右增加(NaBO为69.8 kcal / mol,ClBO为118.98 kcal / mol)。已经对第二行单硼烷基的键合进行了分析,强调了第二行X–B和X–O键的不同特征。
更新日期:2017-12-27
中文翻译:

单硼烷基化合物的结构趋势:第二行元素与BO相互作用的分析
对第二行元素的单硼烷基化合物[XBO](X = Na,Si,P,S,Cl)进行了理论研究。可以观察到,当移动到周期的右侧时,对XBO设置的偏好更高。在单硼酸钠钠中,三个极小值的特征都非常接近:线性NaBO,线性NaOB和L形结构。线性NaBO和L形结构几乎是等能量的,而线性NaOB位于线性NaBO之上2.11 kcal / mol。L形结构转化为线性NaBO的势垒约为5.1 kcal / mol,表明这两个物种都可能成为实验检测的潜在目标。对于单硼硅硅,发现了两个最小值,线性SiBO和线性SiOB,后者比SiBO高约13 kcal / mol。SiOB异构化为SiBO的势垒估计为11.4 kcal / mol。对于磷,硫和氯单硼烷基而言,线性XBO异构体显然是最稳定的异构体,而且转化为XOB物种的障碍也相对较高,这表明线性XBO异构体很可能应该是主要的实验目标。所有研究的单硼烷醇都相对稳定,解离能从第二行的左向右增加(NaBO为69.8 kcal / mol,ClBO为118.98 kcal / mol)。已经对第二行单硼烷基的键合进行了分析,强调了第二行X–B和X–O键的不同特征。并且转化为XOB物种的障碍相对较高,这表明线性XBO异构体很可能应该是主要的实验目标。所有研究的单硼烷醇都相对稳定,解离能从第二行的左向右增加(NaBO为69.8 kcal / mol,ClBO为118.98 kcal / mol)。已经对第二行单硼烷基的键合进行了分析,强调了第二行X–B和X–O键的不同特征。并且转化为XOB物种的障碍相对较高,这表明线性XBO异构体很可能应该是主要的实验目标。所有研究的单硼烷醇都相对稳定,解离能从第二行的左向右增加(NaBO为69.8 kcal / mol,ClBO为118.98 kcal / mol)。已经对第二行单硼烷基的键合进行了分析,强调了第二行X–B和X–O键的不同特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号