Joule ( IF 38.6 ) Pub Date : 2017-12-11 , DOI: 10.1016/j.joule.2017.11.009 Quan Pang , Xiao Liang , Abhinandan Shyamsunder , Linda F. Nazar
|
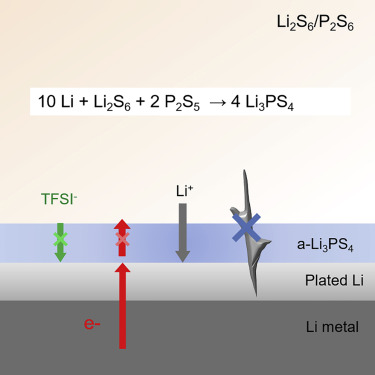
We describe an efficient yet facile strategy to stabilize Li plating by forming a single Li+-ion solid electrolyte layer in vivo on the Li surface using a rationally designed electrolyte additive. This amorphous, homogeneous layer not only reduces the direct contact and parasitic reactions of Li with the liquid electrolyte but also avoids ion depletion and electric field inhomogeneity at the vicinity of the Li surface, thus eliminating dendrite formation. This is evidenced by a 50-fold lower interfacial charge transfer resistance and an 8-fold longer Sand time in Li|Li symmetric cells. The protection layer maintains chemical and electrochemical stability over repeated plating/stripping cycles. We demonstrate stable Li plating/stripping for 2,500 hr at 1 mA cm−2 in symmetric cells, and efficient Li cycling at high current densities up to 8 mA cm−2. Over 400 cycles were achieved at 5-C rate in cells with a Li4Ti5O12 counter electrode at close to 100% coulombic efficiency.
中文翻译:

一种在体内形成固体电解质表层使利金属的电镀稳定
我们描述了一种有效而又简便的策略,通过使用合理设计的电解质添加剂在Li表面上在体内形成单个Li +离子固体电解质层来稳定Li镀层。该非晶均匀层不仅减少了Li与液体电解质的直接接触和寄生反应,而且避免了Li表面附近的离子耗竭和电场不均匀,从而消除了枝晶的形成。在Li | Li对称电池中,界面电荷转移阻力降低了50倍,沙化时间延长了8倍,证明了这一点。保护层在重复的电镀/剥离循环中保持化学和电化学稳定性。我们证明了在1 mA cm -2的条件下进行2500小时稳定的Li镀/剥离在对称电池中,并且在高达8 mA cm -2的高电流密度下有效的Li循环。使用Li 4 Ti 5 O 12对电极以接近100%的库仑效率,以5 C的速率在电池中实现了超过400个循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号