当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Characterization of Human Peptide/Histidine Transporter 1 in Stably Transfected MDCK Cells
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00728 Feifeng Song 1 , Yongjun Hu 2 , Yuqing Wang 1 , David E. Smith 2 , Huidi Jiang 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00728 Feifeng Song 1 , Yongjun Hu 2 , Yuqing Wang 1 , David E. Smith 2 , Huidi Jiang 1
Affiliation
|
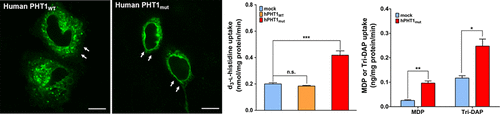
The proton-coupled oligopeptide transporter PHT1 (SLC15A4), which facilitates cross-membrane transport of histidine and small peptides from inside the endosomes or lysosomes to cytosol, plays an important role in intracellular peptides homeostasis and innate immune responses. However, it remains a challenge to elucidate functional properties of the PHT1 transporter because of its subcellular localization. The purpose of this study was to resort hPHT1 protein from the subcellular to outer cell membrane of MDCK cells stably transfected with human PHT1 mutants, and to characterize its functional activity in these cells. Using this model, the functional activity of hPHT1 was evaluated by cellular uptake studies with d3-l-histidine, GlySar, and the bacterial peptidoglycan products MDP and Tri-DAP. We found that the disruption of two dileucine motifs was indispensable for hPHT1 transporter being preferentially targeting to plasma membranes. hPHT1 showed high affinity for d3-l-histidine and low affinity for GlySar, with Km values of 16.3 ± 1.9 μM and 1.60 ± 0.30 mM, respectively. Moreover, the bacterial peptidoglycan components MDP and Tri-DAP were shown conclusively to be hPHT1 substrates. The uptake of MDP by hPHT1 was inhibited by di/tripeptides and peptide-like drugs, but not by glycine and acyclovir. The functional activity of hPHT1 was also pH-dependent, with an optimal cellular uptake in buffer pH 6.5. Taken together, we established a novel cell model to evaluate the function of hPHT1 in vitro, and confirmed that MDP and Tri-DAP were substrates of hPHT1. Our findings suggest that PHT1 may serve as a potential target for reducing the immune responses and for drug treatment of inflammatory diseases.
中文翻译:

人肽/组氨酸转运蛋白1在稳定转染的MDCK细胞中的功能表征
质子偶联的寡肽转运蛋白PHT1(SLC15A4)促进组氨酸和小肽从内体或溶酶体内部跨膜转运到胞质溶胶,在细胞内肽稳态和先天免疫应答中起重要作用。然而,由于其亚细胞定位,阐明PHT1转运蛋白的功能特性仍然是一个挑战。这项研究的目的是将hPHT1蛋白从被人PHT1突变体稳定转染的MDCK细胞的亚细胞膜转移到细胞外膜,并表征其在这些细胞中的功能活性。使用该模型,通过d 3 - l的细胞摄取研究评估了hPHT1的功能活性-组氨酸,GlySar和细菌肽聚糖产品MDP和Tri-DAP。我们发现破坏两个双亮氨酸基序对于hPHT1转运蛋白优先靶向质膜是必不可少的。hPHT1显示为d的高亲和力3 -升-组氨酸和低亲和力GlySar,与ķ米分别为16.3±1.9μM和1.60±0.30 mM。此外,细菌肽聚糖成分MDP和Tri-DAP最终显示为hPHT1底物。hPHT1对MDP的吸收被二肽/三肽和类似肽的药物抑制,但不受甘氨酸和阿昔洛韦的抑制。hPHT1的功能活性也与pH有关,在pH 6.5的缓冲液中具有最佳的细胞摄取。两者合计,我们建立了一个新的细胞模型,以评估hPHT1的体外功能,并证实MDP和Tri-DAP是hPHT1的底物。我们的发现表明,PHT1可能作为降低免疫反应和治疗炎症性疾病的潜在靶标。
更新日期:2018-01-02
中文翻译:

人肽/组氨酸转运蛋白1在稳定转染的MDCK细胞中的功能表征
质子偶联的寡肽转运蛋白PHT1(SLC15A4)促进组氨酸和小肽从内体或溶酶体内部跨膜转运到胞质溶胶,在细胞内肽稳态和先天免疫应答中起重要作用。然而,由于其亚细胞定位,阐明PHT1转运蛋白的功能特性仍然是一个挑战。这项研究的目的是将hPHT1蛋白从被人PHT1突变体稳定转染的MDCK细胞的亚细胞膜转移到细胞外膜,并表征其在这些细胞中的功能活性。使用该模型,通过d 3 - l的细胞摄取研究评估了hPHT1的功能活性-组氨酸,GlySar和细菌肽聚糖产品MDP和Tri-DAP。我们发现破坏两个双亮氨酸基序对于hPHT1转运蛋白优先靶向质膜是必不可少的。hPHT1显示为d的高亲和力3 -升-组氨酸和低亲和力GlySar,与ķ米分别为16.3±1.9μM和1.60±0.30 mM。此外,细菌肽聚糖成分MDP和Tri-DAP最终显示为hPHT1底物。hPHT1对MDP的吸收被二肽/三肽和类似肽的药物抑制,但不受甘氨酸和阿昔洛韦的抑制。hPHT1的功能活性也与pH有关,在pH 6.5的缓冲液中具有最佳的细胞摄取。两者合计,我们建立了一个新的细胞模型,以评估hPHT1的体外功能,并证实MDP和Tri-DAP是hPHT1的底物。我们的发现表明,PHT1可能作为降低免疫反应和治疗炎症性疾病的潜在靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号