当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allenamide as a bioisostere of acrylamide in the design and synthesis of targeted covalent inhibitors†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1039/c7md00571g Deheng Chen 1, 2, 3, 4, 5 , Dexiang Guo 1, 2, 3, 4, 5 , Ziqin Yan 1, 2, 3, 4, 5 , Yujun Zhao 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1039/c7md00571g Deheng Chen 1, 2, 3, 4, 5 , Dexiang Guo 1, 2, 3, 4, 5 , Ziqin Yan 1, 2, 3, 4, 5 , Yujun Zhao 1, 2, 3, 4, 5
Affiliation
|
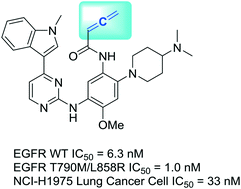
The success of acrylamide-containing drugs in treating cancers has spurred a passion to search for acrylamide bioisosteres. In our endeavour, we have identified that an allenamide group can be a reactive bioisostere of the acrylamide group. In our development of allenamide-containing compounds, we found that the most potent compound, 14, inhibited the kinase activities of both T790M/L858R double mutant and wild type EGFR in a low nM range. 14 also inhibited the growth of NCI-H1975 lung cancer cells at IC50 = 33 nM, which is comparable to that of acrylamide-containing osimertinib. The western blot analysis showed that the phosphorylation of EGFR, AKT, and ERK1/2 was simultaneously inhibited in a dose-dependent manner when NCI-H1975 cells were treated with 14. By measuring the conjugate addition product formed by 14 and GSH, we obtained a reaction rate constant of 302.5 × 10−3 min−1, which is about 30-fold higher than that of osimertinib. Taken together, our data suggest that the allenamide-containing compounds inhibited EGFR kinases through covalent modifications. Our study indicates that the allenamide group could serve as an alternative electrophilic warhead in the design of targeted covalent inhibitors, and this bioisostere replacement may have broad applications in medicinal chemistry.
中文翻译:

Allenamide作为丙烯酰胺的生物等排体,可用于目标共价抑制剂的设计和合成†
含丙烯酰胺的药物在治疗癌症方面的成功激发了人们对寻找丙烯酰胺生物异构体的热情。在我们的努力中,我们已经发现烯丙酰胺基团可以是丙烯酰胺基团的反应性生物等排体。在开发含烯丙酰胺的化合物时,我们发现最有效的化合物14在低nM范围内抑制T790M / L858R双突变体和野生型EGFR的激酶活性。图14还抑制了NCI-H1975肺癌细胞在IC 50 = 33 nM时的生长,这与含丙烯酰胺的奥西替尼相当。Western印迹分析表明,当用NCI-H1975细胞处理EGFR,AKT和ERK1 / 2的磷酸化时,剂量依赖性同时抑制了EGFR,AKT和ERK1 / 2的磷酸化。14。通过测量由14和GSH形成的结合物加成产物,我们获得了302.5×10 -3 min -1的反应速率常数,比奥西替尼高约30倍。两者合计,我们的数据表明,含Allenamide的化合物通过共价修饰抑制EGFR激酶。我们的研究表明,烯丙酰胺基团可以在靶向共价抑制剂的设计中用作替代的亲电子弹头,这种生物等排体替代物可能在药物化学中具有广泛的应用。
更新日期:2017-12-11
中文翻译:

Allenamide作为丙烯酰胺的生物等排体,可用于目标共价抑制剂的设计和合成†
含丙烯酰胺的药物在治疗癌症方面的成功激发了人们对寻找丙烯酰胺生物异构体的热情。在我们的努力中,我们已经发现烯丙酰胺基团可以是丙烯酰胺基团的反应性生物等排体。在开发含烯丙酰胺的化合物时,我们发现最有效的化合物14在低nM范围内抑制T790M / L858R双突变体和野生型EGFR的激酶活性。图14还抑制了NCI-H1975肺癌细胞在IC 50 = 33 nM时的生长,这与含丙烯酰胺的奥西替尼相当。Western印迹分析表明,当用NCI-H1975细胞处理EGFR,AKT和ERK1 / 2的磷酸化时,剂量依赖性同时抑制了EGFR,AKT和ERK1 / 2的磷酸化。14。通过测量由14和GSH形成的结合物加成产物,我们获得了302.5×10 -3 min -1的反应速率常数,比奥西替尼高约30倍。两者合计,我们的数据表明,含Allenamide的化合物通过共价修饰抑制EGFR激酶。我们的研究表明,烯丙酰胺基团可以在靶向共价抑制剂的设计中用作替代的亲电子弹头,这种生物等排体替代物可能在药物化学中具有广泛的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号