当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the structural stability of LiBH4 through boron-based compounds towards superior dehydrogenation†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1039/c7ta09376d Weitong Cai 1, 2, 3, 4 , Juner Chen 4, 5, 6, 7 , Liying Liu 1, 2, 3, 4 , Yuanzheng Yang 1, 2, 3, 4 , Hui Wang 4, 8, 9, 10, 11
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1039/c7ta09376d Weitong Cai 1, 2, 3, 4 , Juner Chen 4, 5, 6, 7 , Liying Liu 1, 2, 3, 4 , Yuanzheng Yang 1, 2, 3, 4 , Hui Wang 4, 8, 9, 10, 11
Affiliation
|
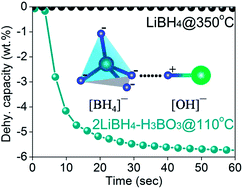
The remarkable destabilization effects of H3BO3, HBO2, and B2O3 on dehydrogenation of LiBH4 are revealed in this work. The effectiveness of destabilizing the structural stability is in the order of H3BO3 > HBO2 > B2O3. Besides, through optimizing the molar ratio of LiBH4 and H3BO3 and milling treatment, the destabilization effect, especially for dehydrogenation kinetics, is further enhanced. For example, at a temperature as low as 110 °C, 5.8 wt% hydrogen can be liberated in seconds from 2LiBH4–H3BO3 prepared through pre-milling. The investigation reveals that each of the LiBH4–H3BO3, LiBH4–HBO2 and LiBH4–B2O3 systems undergo multiple dehydrogenation stages corresponding to different destabilization mechanisms. The reaction at lower temperature is ascribed to the H+⋯H− coupling mechanism which should be enhanced by the [OH]−⋯[BH4]− interaction mode. Pre-milling treatment of LiBH4 and H3BO3 also promotes the H+⋯H− interaction which may have originated from the increasing contact area as a result of the fine particles, and therefore probably reduced the reaction activation energy. Consequently, it gives rise to the superior dehydrogenation performance of lower temperature, rapid kinetics, pure hydrogen and high capacity, which are required for off-board hydrogen energy vehicle application.
中文翻译:

通过硼基化合物 调节LiBH 4的结构稳定性,以实现出色的脱氢作用†
这项工作揭示了H 3 BO 3,HBO 2和B 2 O 3对LiBH 4脱氢的显着去稳定作用。使结构稳定性不稳定的有效性按H 3 BO 3 > HBO 2 > B 2 O 3的顺序排列。此外,通过优化LiBH 4与H 3 BO 3的摩尔比在研磨处理中,去稳定作用,特别是对于脱氢动力学的去稳定作用被进一步增强。例如,在低至110°C的温度下,可以在几秒钟内从通过预研磨制备的2LiBH 4 -H 3 BO 3中释放出5.8 wt%的氢。研究表明,LiBH 4 -H 3 BO 3,LiBH 4 -HBO 2和LiBH 4 -B 2 O 3系统分别经历了多个脱氢阶段,分别对应不同的去稳定机理。在较低的温度下进行反应是归因到H + ⋯ħ -[OH] - ⋯[BH 4 ] -相互作用模式应增强的耦合机理。的LiBH的预研磨处理4和H 3 BO 3还促进为H + ⋯ħ -这可能源自增大的接触面积作为细颗粒的结果,并因此可能降低了反应活化能的相互作用。因此,它产生了低温,快速动力学,纯氢和高容量等优异的脱氢性能,这是车外氢能汽车应用所需的。
更新日期:2017-12-11
中文翻译:

通过硼基化合物 调节LiBH 4的结构稳定性,以实现出色的脱氢作用†
这项工作揭示了H 3 BO 3,HBO 2和B 2 O 3对LiBH 4脱氢的显着去稳定作用。使结构稳定性不稳定的有效性按H 3 BO 3 > HBO 2 > B 2 O 3的顺序排列。此外,通过优化LiBH 4与H 3 BO 3的摩尔比在研磨处理中,去稳定作用,特别是对于脱氢动力学的去稳定作用被进一步增强。例如,在低至110°C的温度下,可以在几秒钟内从通过预研磨制备的2LiBH 4 -H 3 BO 3中释放出5.8 wt%的氢。研究表明,LiBH 4 -H 3 BO 3,LiBH 4 -HBO 2和LiBH 4 -B 2 O 3系统分别经历了多个脱氢阶段,分别对应不同的去稳定机理。在较低的温度下进行反应是归因到H + ⋯ħ -[OH] - ⋯[BH 4 ] -相互作用模式应增强的耦合机理。的LiBH的预研磨处理4和H 3 BO 3还促进为H + ⋯ħ -这可能源自增大的接触面积作为细颗粒的结果,并因此可能降低了反应活化能的相互作用。因此,它产生了低温,快速动力学,纯氢和高容量等优异的脱氢性能,这是车外氢能汽车应用所需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号