当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Peptidomimetic Antibody–Drug Conjugate Linkers with Enhanced Protease Specificity
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01430 BinQing Wei 1 , Janet Gunzner-Toste 1 , Hui Yao 2 , Tao Wang 2 , Jing Wang 2 , Zijin Xu 2 , Jinhua Chen 2 , John Wai 2 , Jim Nonomiya 1 , Siao Ping Tsai 1 , Josefa Chuh 1 , Katherine R. Kozak 1 , Yichin Liu 1 , Shang-Fan Yu 1 , Jeff Lau 1 , Guangmin Li 1 , Gail D. Phillips 1 , Doug Leipold 1 , Amrita Kamath 1 , Dian Su 1 , Keyang Xu 1 , Charles Eigenbrot 1 , Stefan Steinbacher 3 , Rachana Ohri 1 , Helga Raab 1 , Leanna R. Staben 1 , Guiling Zhao 1 , John A. Flygare 1 , Thomas H. Pillow 1 , Vishal Verma 1 , Luke A. Masterson 4 , Philip W. Howard 4 , Brian Safina 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01430 BinQing Wei 1 , Janet Gunzner-Toste 1 , Hui Yao 2 , Tao Wang 2 , Jing Wang 2 , Zijin Xu 2 , Jinhua Chen 2 , John Wai 2 , Jim Nonomiya 1 , Siao Ping Tsai 1 , Josefa Chuh 1 , Katherine R. Kozak 1 , Yichin Liu 1 , Shang-Fan Yu 1 , Jeff Lau 1 , Guangmin Li 1 , Gail D. Phillips 1 , Doug Leipold 1 , Amrita Kamath 1 , Dian Su 1 , Keyang Xu 1 , Charles Eigenbrot 1 , Stefan Steinbacher 3 , Rachana Ohri 1 , Helga Raab 1 , Leanna R. Staben 1 , Guiling Zhao 1 , John A. Flygare 1 , Thomas H. Pillow 1 , Vishal Verma 1 , Luke A. Masterson 4 , Philip W. Howard 4 , Brian Safina 1
Affiliation
|
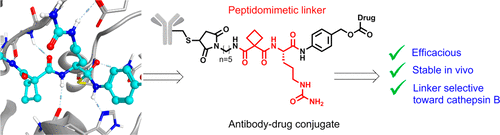
Antibody–drug conjugates (ADCs) have become an important therapeutic modality for oncology, with three approved by the FDA and over 60 others in clinical trials. Despite the progress, improvements in ADC therapeutic index are desired. Peptide-based ADC linkers that are cleaved by lysosomal proteases have shown sufficient stability in serum and effective payload-release in targeted cells. If the linker can be preferentially hydrolyzed by tumor-specific proteases, safety margin may improve. However, the use of peptide-based linkers limits our ability to modulate protease specificity. Here we report the structure-guided discovery of novel, nonpeptidic ADC linkers. We show that a cyclobutane-1,1-dicarboxamide-containing linker is hydrolyzed predominantly by cathepsin B while the valine–citrulline dipeptide linker is not. ADCs bearing the nonpeptidic linker are as efficacious and stable in vivo as those with the dipeptide linker. Our results strongly support the application of the peptidomimetic linker and present new opportunities for improving the selectivity of ADCs.
中文翻译:

拟肽抗体-药物缀合物接头具有增强的蛋白酶特异性
抗体-药物偶联物(ADC)已成为肿瘤学的重要治疗手段,其中三种已获得FDA批准,另外60多项已在临床试验中获得批准。尽管取得了进步,但仍需要改善ADC治疗指数。被溶酶体蛋白酶裂解的基于肽的ADC接头在血清中显示出足够的稳定性,并在靶细胞中有效释放有效负载。如果该接头可以被肿瘤特异性蛋白酶优先水解,则安全系数可能会提高。但是,基于肽的接头的使用限制了我们调节蛋白酶特异性的能力。在这里,我们报告新型非肽类ADC接头的结构指导发现。我们表明,含有环丁烷-1,1-二羧酸的连接子主要被组织蛋白酶B水解,而缬氨酸-瓜氨酸二肽连接子则不被水解。带有非肽接头的ADC在体内与具有二肽接头的ADC一样有效和稳定。我们的结果有力地支持了拟肽连接子的应用,并为改善ADC的选择性提供了新的机会。
更新日期:2017-12-21
中文翻译:

拟肽抗体-药物缀合物接头具有增强的蛋白酶特异性
抗体-药物偶联物(ADC)已成为肿瘤学的重要治疗手段,其中三种已获得FDA批准,另外60多项已在临床试验中获得批准。尽管取得了进步,但仍需要改善ADC治疗指数。被溶酶体蛋白酶裂解的基于肽的ADC接头在血清中显示出足够的稳定性,并在靶细胞中有效释放有效负载。如果该接头可以被肿瘤特异性蛋白酶优先水解,则安全系数可能会提高。但是,基于肽的接头的使用限制了我们调节蛋白酶特异性的能力。在这里,我们报告新型非肽类ADC接头的结构指导发现。我们表明,含有环丁烷-1,1-二羧酸的连接子主要被组织蛋白酶B水解,而缬氨酸-瓜氨酸二肽连接子则不被水解。带有非肽接头的ADC在体内与具有二肽接头的ADC一样有效和稳定。我们的结果有力地支持了拟肽连接子的应用,并为改善ADC的选择性提供了新的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号