当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strong electronic coupling of molecular sites to graphitic electrodes via pyrazine conjugation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-16 , DOI: 10.1021/jacs.7b10723 Megan N. Jackson 1 , Seokjoon Oh 1 , Corey J. Kaminsky 1 , Sterling B. Chu 1 , Guanghui Zhang 2 , Jeffrey T. Miller 2, 3 , Yogesh Surendranath 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-16 , DOI: 10.1021/jacs.7b10723 Megan N. Jackson 1 , Seokjoon Oh 1 , Corey J. Kaminsky 1 , Sterling B. Chu 1 , Guanghui Zhang 2 , Jeffrey T. Miller 2, 3 , Yogesh Surendranath 1
Affiliation
|
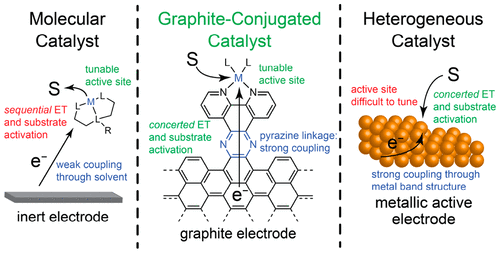
Glassy carbon electrodes were functionalized with redox-active moieties by condensation of o-phenylenediamine derivatives with o-quinone sites native to graphitic carbon surfaces. Electrochemical and spectroscopic investigations establish that these graphite-conjugated catalysts (GCCs) exhibit strong electronic coupling to the electrode, leading to electron transfer (ET) behavior that diverges fundamentally from that of solution-phase or surface-tethered analogues. We find that (1) ET is not observed between the electrode and a redox-active GCC moiety regardless of applied potential. (2) ET is observed at GCCs only if the interfacial reaction is ion-coupled. (3) Even when ET is observed, the oxidation state of a transition metal GCC site remains unchanged. From these observations, we construct a mechanistic model for GCC sites in which ET behavior is identical to that of catalytically active metal surfaces rather than to that of molecules in solution. These results suggest that GCCs provide a versatile platform for bridging molecular and heterogeneous electrocatalysis.
中文翻译:

分子位点通过吡嗪共轭与石墨电极的强电子耦合
通过邻苯二胺衍生物与石墨碳表面天然的邻醌位点缩合,玻璃碳电极被氧化还原活性部分功能化。电化学和光谱研究表明,这些石墨共轭催化剂 (GCC) 表现出与电极的强电子耦合,导致电子转移 (ET) 行为从根本上不同于溶液相或表面束缚类似物的行为。我们发现 (1) ET 在电极和氧化还原活性 GCC 部分之间没有观察到,无论施加的电位如何。(2) 只有当界面反应是离子耦合时,才会在 GCC 上观察到 ET。(3) 即使观察到 ET,过渡金属 GCC 位点的氧化态也保持不变。从这些观察中,我们为 GCC 位点构建了一个机械模型,其中 ET 行为与催化活性金属表面的行为相同,而不是与溶液中分子的行为相同。这些结果表明 GCC 为桥接分子和多相电催化提供了一个通用平台。
更新日期:2018-01-16
中文翻译:

分子位点通过吡嗪共轭与石墨电极的强电子耦合
通过邻苯二胺衍生物与石墨碳表面天然的邻醌位点缩合,玻璃碳电极被氧化还原活性部分功能化。电化学和光谱研究表明,这些石墨共轭催化剂 (GCC) 表现出与电极的强电子耦合,导致电子转移 (ET) 行为从根本上不同于溶液相或表面束缚类似物的行为。我们发现 (1) ET 在电极和氧化还原活性 GCC 部分之间没有观察到,无论施加的电位如何。(2) 只有当界面反应是离子耦合时,才会在 GCC 上观察到 ET。(3) 即使观察到 ET,过渡金属 GCC 位点的氧化态也保持不变。从这些观察中,我们为 GCC 位点构建了一个机械模型,其中 ET 行为与催化活性金属表面的行为相同,而不是与溶液中分子的行为相同。这些结果表明 GCC 为桥接分子和多相电催化提供了一个通用平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号