当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and characterization of pyrrolyldipyrrin F-BODIPYs†
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1039/c7pp00341b Sarah M. Greening 1, 2, 3, 4 , Katherine N. Robertson 1, 3, 4, 5 , Alison Thompson 1, 2, 3, 4
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1039/c7pp00341b Sarah M. Greening 1, 2, 3, 4 , Katherine N. Robertson 1, 3, 4, 5 , Alison Thompson 1, 2, 3, 4
Affiliation
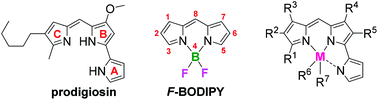
|
A series of synthetic analogs of the tripyrrolic natural product prodigiosin were complexed with boron trifluoride to generate the corresponding F-BODIPYs. The maximum wavelengths of absorption and emission of the pyrrolyldipyrrin F-BODIPYs were tuned through variation of the substituents about the pyrrolyldipyrrinato core. The limited variation of substituents on the C-ring did not significantly affect absorption and emission. However, variation of substituents on the B-ring and A-ring resulted in a corresponding red-shift in absorption and emission reaching maximum wavelengths of 600 nm. The presence of electron donating substituents on the B-ring caused a decrease in the Stokes shift, while the presence of electron-withdrawing substituents caused an increase, ranging from 3–25 nm. Stokes shifts were solvent-dependant for some compounds. The inclusion of a dimethylamino group resulted in photo-induced electron transfer and thus quenched fluorescence which was restored upon protonation.
中文翻译:

合成和pyrrolyldipyrrin表征˚F -BODIPYs †
将一系列三吡咯天然产物prodigiosin的合成类似物与三氟化硼复合以生成相应的F -BODIPY。吡咯基联吡啶F的最大吸收和发射波长通过改变围绕吡咯基二吡喃酮核心的取代基来调节-BODIPY。C环上取代基的有限变化不会显着影响吸收和发射。但是,B环和A环上取代基的变化导致吸收和发射的相应红移达到600 nm的最大波长。B环上给电子取代基的存在导致斯托克斯位移降低,而吸电子取代基的存在引起3-25 nm范围的增加。对于某些化合物,斯托克斯位移取决于溶剂。包含二甲基氨基导致光诱导的电子转移,并因此猝灭了荧光,其在质子化时得以恢复。
更新日期:2017-12-08
中文翻译:

合成和pyrrolyldipyrrin表征˚F -BODIPYs †
将一系列三吡咯天然产物prodigiosin的合成类似物与三氟化硼复合以生成相应的F -BODIPY。吡咯基联吡啶F的最大吸收和发射波长通过改变围绕吡咯基二吡喃酮核心的取代基来调节-BODIPY。C环上取代基的有限变化不会显着影响吸收和发射。但是,B环和A环上取代基的变化导致吸收和发射的相应红移达到600 nm的最大波长。B环上给电子取代基的存在导致斯托克斯位移降低,而吸电子取代基的存在引起3-25 nm范围的增加。对于某些化合物,斯托克斯位移取决于溶剂。包含二甲基氨基导致光诱导的电子转移,并因此猝灭了荧光,其在质子化时得以恢复。











































 京公网安备 11010802027423号
京公网安备 11010802027423号