当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Heterocycle-Ligated Borocations as Highly Tunable Carbon Lewis Acids.
Organometallics ( IF 2.5 ) Pub Date : 2017-12-08 , DOI: 10.1021/acs.organomet.7b00779 James E Radcliffe 1 , Jay J Dunsford 1 , Jessica Cid 1 , Valerio Fasano 1 , Michael J Ingleson 1
Organometallics ( IF 2.5 ) Pub Date : 2017-12-08 , DOI: 10.1021/acs.organomet.7b00779 James E Radcliffe 1 , Jay J Dunsford 1 , Jessica Cid 1 , Valerio Fasano 1 , Michael J Ingleson 1
Affiliation
|
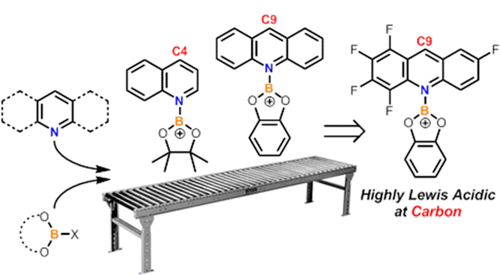
The relative (to BEt3) hydride ion affinity (HIA) of a series of acridine borenium salts has been calculated, with some HIAs found to be similar to that for [Ph3C]+. The HIA at the acridine C9 position is controlled by both acridine and the boron substituents, the latter presumably affecting the strength of the B=N bond in the acridane-BY2 products from hydride transfer. Through a range of hydride abstraction benchmarking reactions against organic hydride donors the experimental HIA of [F5acr-BCat]+ (cat = catechol, F5acr = 1,2,3,4,7-pentafluoroacridine) has been confirmed to be extremely high and closely comparable to that of [Ph3C]+. The high HIA of [F5acr-BCat]+ enables H2 and alkene activation in a FLP with 2,6-di-tert-butylpyridine. Finally, the HIA of pyridine and quinoline borenium cations has been determined, with the HIA at boron in [PinB(amine)]+ (pin = pinacol, amine = pyridine or quinoline) found to be relatively low. This enabled the hydroboration of pyridine and quinoline by HBPin to be achieved through the addition of 5-10 mol % of bench-stable cationic carbon Lewis acids such as 2-phenyl-N,N-dimethylimidazolium salts.
中文翻译:

N-杂环连接的硼酸作为高度可调的碳路易斯酸。
已计算出一系列a啶硼鎓盐的相对(相对于BEt3)氢化物离子亲和力(HIA),发现一些HIA与[Ph3C] +相似。9啶C9位置的HIA受a啶和硼取代基的控制,后者可能会影响氢化物转移引起的cri啶BY2产品中B = N键的强度。通过一系列针对有机氢化物供体的氢化物提取基准反应,已证实[F5acr-BCat] +(cat =儿茶酚,F5acr = 1,2,3,4,7-五氟ac啶)的实验HIA非常高且非常接近与[Ph3C] +相当。[F5acr-BCat] +的高HIA使FLP中带有2,6-二叔丁基吡啶的H2和烯烃活化成为可能。最后,确定了吡啶和喹啉硼阳离子的HIA,发现[PinB(胺)] +(pin =频哪醇,胺=吡啶或喹啉)中硼的HIA相对较低。这使得能够通过添加5-10mol%的台式稳定的阳离子碳路易斯酸例如2-苯基-N,N-二甲基咪唑鎓盐来实现HBPin对吡啶和喹啉的硼氢化。
更新日期:2017-12-10
中文翻译:

N-杂环连接的硼酸作为高度可调的碳路易斯酸。
已计算出一系列a啶硼鎓盐的相对(相对于BEt3)氢化物离子亲和力(HIA),发现一些HIA与[Ph3C] +相似。9啶C9位置的HIA受a啶和硼取代基的控制,后者可能会影响氢化物转移引起的cri啶BY2产品中B = N键的强度。通过一系列针对有机氢化物供体的氢化物提取基准反应,已证实[F5acr-BCat] +(cat =儿茶酚,F5acr = 1,2,3,4,7-五氟ac啶)的实验HIA非常高且非常接近与[Ph3C] +相当。[F5acr-BCat] +的高HIA使FLP中带有2,6-二叔丁基吡啶的H2和烯烃活化成为可能。最后,确定了吡啶和喹啉硼阳离子的HIA,发现[PinB(胺)] +(pin =频哪醇,胺=吡啶或喹啉)中硼的HIA相对较低。这使得能够通过添加5-10mol%的台式稳定的阳离子碳路易斯酸例如2-苯基-N,N-二甲基咪唑鎓盐来实现HBPin对吡啶和喹啉的硼氢化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号