当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Securamine Derivatives from the Arctic Bryozoan Securiflustra securifrons
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00703 Kine Ø. Hansen 1 , Johan Isaksson 2 , Annette Bayer 2 , Jostein A. Johansen 2 , Jeanette H. Andersen 1 , Espen Hansen 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00703 Kine Ø. Hansen 1 , Johan Isaksson 2 , Annette Bayer 2 , Jostein A. Johansen 2 , Jeanette H. Andersen 1 , Espen Hansen 1
Affiliation
|
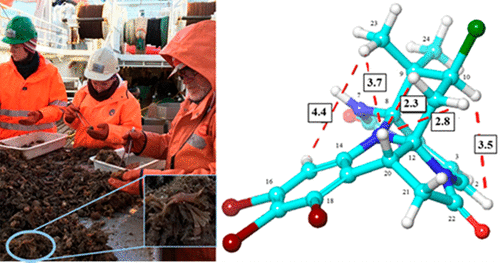
Bryozoans belonging to the Flustridae family have proven to be a rich source of structurally unique secondary metabolites. As part of our continuing search for bioactive secondary metabolites from Arctic marine invertebrates, the organic extract of Securiflustra securifrons was examined. This resulted in the isolation of three new halogenated, hexacyclic indole-imidazole alkaloids, securamines H–J (1–3), together with the previously reported compounds securamines C (4) and E (5). The structures of the new compounds were elucidated by spectroscopic methods including 1D and 2D NMR and analysis of HRMS data. Through NMR and HRMS analysis, we were also able to prove that 1, 2, 4, and 5, when dissolved in MeOH, were converted into their corresponding artifacts, the securamine MeOH adducts m1, m2, m4, and m5. When redissolved in a non-nucleophilic solvent, the native variants were re-formed. We also found that 3 was a MeOH addition product of a native variant. Even though the structures of several securamines have been reported, their bioactivities were not examined. The securamines displayed various degrees of cytotoxicity against the human cancer cell lines A2058 (skin), HT-29 (colon), and MCF-7 (breast), as well as against nonmalignant human MRC-5 lung fibroblasts. Compounds 1, 2, and 5 were found to be active, with IC50 values against the cancer cell lines ranging from 1.4 ± 0.1 to 10 ± 1 μM. The cytotoxicity of 1 was further evaluated and found to be time-dependent.
中文翻译:

北极苔藓菌Securiflustra securifrons的Securamine衍生物
事实证明,属于Flustridae家族的苔藓虫是丰富的结构独特的次级代谢产物来源。在我们不断寻找北极无脊椎动物生物活性次生代谢产物的过程中,对Securiflustra securifrons的有机提取物进行了检查。这导致了三个新的卤化的,六环吲哚生物碱咪唑隔离,securamines H-Ĵ(1 - 3),连同先前报道化合物securamines C(4)和E(5)。通过包括1D和2D NMR的光谱方法以及HRMS数据分析,阐明了新化合物的结构。通过NMR和HRMS分析,我们还能够证明1,2,4,和5,溶于MeOH的情况下,被转换成其相应的伪影,所述securamine甲醇加合物M1,M2,M4,和M5。当重新溶解在非亲核溶剂中时,天然变体重新形成。我们还发现3是天然变异体的MeOH加成产物。即使已经报道了几种securamines的结构,但未检查其生物活性。securamines对人类癌细胞系A2058(皮肤),HT-29(结肠)和MCF-7(乳腺)以及对非恶性人类MRC-5肺成纤维细胞显示出不同程度的细胞毒性。化合物1,发现2和5具有活性,针对癌细胞系的IC 50值为1.4±0.1至10±1μM。进一步评估了1的细胞毒性,发现它是时间依赖性的。
更新日期:2017-12-08
中文翻译:

北极苔藓菌Securiflustra securifrons的Securamine衍生物
事实证明,属于Flustridae家族的苔藓虫是丰富的结构独特的次级代谢产物来源。在我们不断寻找北极无脊椎动物生物活性次生代谢产物的过程中,对Securiflustra securifrons的有机提取物进行了检查。这导致了三个新的卤化的,六环吲哚生物碱咪唑隔离,securamines H-Ĵ(1 - 3),连同先前报道化合物securamines C(4)和E(5)。通过包括1D和2D NMR的光谱方法以及HRMS数据分析,阐明了新化合物的结构。通过NMR和HRMS分析,我们还能够证明1,2,4,和5,溶于MeOH的情况下,被转换成其相应的伪影,所述securamine甲醇加合物M1,M2,M4,和M5。当重新溶解在非亲核溶剂中时,天然变体重新形成。我们还发现3是天然变异体的MeOH加成产物。即使已经报道了几种securamines的结构,但未检查其生物活性。securamines对人类癌细胞系A2058(皮肤),HT-29(结肠)和MCF-7(乳腺)以及对非恶性人类MRC-5肺成纤维细胞显示出不同程度的细胞毒性。化合物1,发现2和5具有活性,针对癌细胞系的IC 50值为1.4±0.1至10±1μM。进一步评估了1的细胞毒性,发现它是时间依赖性的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号