当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of Acinetobacter-Derived Cephalosporinase: Exploring the Carboxylate Recognition Site Using Novel β-Lactamase Inhibitors
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acsinfecdis.7b00153 Emilia Caselli 1 , Chiara Romagnoli 1 , Rachel A. Powers 2 , Magdalena A. Taracila 3 , Alexandra A. Bouza 2 , Hollister C. Swanson 2 , Kali A. Smolen 2 , Francesco Fini 1 , Bradley J. Wallar 2 , Robert A. Bonomo 3, 4 , Fabio Prati 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acsinfecdis.7b00153 Emilia Caselli 1 , Chiara Romagnoli 1 , Rachel A. Powers 2 , Magdalena A. Taracila 3 , Alexandra A. Bouza 2 , Hollister C. Swanson 2 , Kali A. Smolen 2 , Francesco Fini 1 , Bradley J. Wallar 2 , Robert A. Bonomo 3, 4 , Fabio Prati 1
Affiliation
|
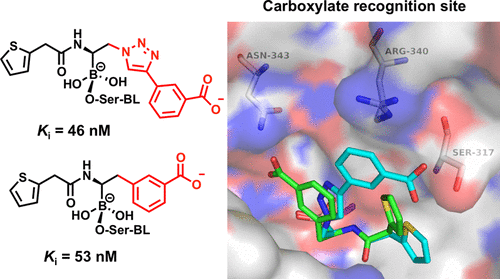
Boronic acids are attracting a lot of attention as β-lactamase inhibitors, and in particular, compound S02030 (Ki = 44 nM) proved to be a good lead compound against ADC-7 (Acinetobacter-derived cephalosporinase), one of the most significant resistance determinants in A. baumannii. The atomic structure of the ADC-7/S02030 complex highlighted the importance of critical structural determinants for recognition of the boronic acids. Herein, to elucidate the role in recognition of the R2-carboxylate, which mimics the C3/C4 found in β-lactams, we designed, synthesized, and characterized six derivatives of S02030 (3a). Out of the six compounds, the best inhibitors proved to be those with an explicit negative charge (compounds 3a–c, 3h, and 3j, Ki = 44–115 nM), which is in contrast to the derivatives where the negative charge is omitted, such as the amide derivative 3d (Ki = 224 nM) and the hydroxyamide derivative 3e (Ki = 155 nM). To develop a structural characterization of inhibitor binding in the active site, the X-ray crystal structures of ADC-7 in a complex with compounds 3c, SM23, and EC04 were determined. All three compounds share the same structural features as in S02030 but only differ in the carboxy-R2 side chain, thereby providing the opportunity of exploring the distinct binding mode of the negatively charged R2 side chain. This cephalosporinase demonstrates a high degree of versatility in recognition, employing different residues to directly interact with the carboxylate, thus suggesting the existence of a “carboxylate binding region” rather than a binding site in ADC enzymes. Furthermore, this class of compounds was tested against resistant clinical strains of A. baumannii and are effective at inhibiting bacterial growth in conjunction with a β-lactam antibiotic.
中文翻译:

抑制不动杆菌衍生头孢菌素:探索羧酸识别网站使用新型β内酰胺酶抑制剂
硼酸作为β-内酰胺酶抑制剂引起了广泛的关注,特别是化合物S02030(K i = 44 nM)被证明是对抗ADC-7(不动杆菌衍生的头孢菌素酶)的良好先导化合物,ADC-7是最重要的化合物之一。鲍曼不动杆菌中的抗性决定因素。ADC-7 / S02030配合物的原子结构突显了关键结构决定因素对于识别硼酸的重要性。在本文中,为了阐明在识别R2-羧酸酯(模拟在β-内酰胺中发现的C 3 / C 4)中的作用,我们设计,合成并表征了S02030的六种衍生物(3a)。在这六种化合物中,最好的抑制剂被证明是带有明显负电荷的化合物(化合物3a – c,3h和3j,K i = 44–115 nM),这与衍生物的负电荷相反。诸如酰胺衍生物3d(K i= 224nM)和羟酰胺衍生物3e(K i= 155nM)被省略。为了开发抑制剂在活性位点结合的结构特征,ADC-7与化合物3c,SM23和EC04的复合物的X射线晶体结构被确定。这三种化合物均具有与S02030相同的结构特征,但仅羧基R2侧链不同,从而为探索带负电荷的R2侧链的独特结合方式提供了机会。这种头孢菌素酶在识别方面表现出高度的多功能性,它采用不同的残基直接与羧酸盐相互作用,因此暗示了ADC酶中存在“羧酸盐结合区”而不是结合位点。此外,该类化合物针对鲍曼不动杆菌的耐药临床菌株进行了测试,并与β-内酰胺类抗生素一起有效抑制细菌生长。
更新日期:2017-11-16
中文翻译:

抑制不动杆菌衍生头孢菌素:探索羧酸识别网站使用新型β内酰胺酶抑制剂
硼酸作为β-内酰胺酶抑制剂引起了广泛的关注,特别是化合物S02030(K i = 44 nM)被证明是对抗ADC-7(不动杆菌衍生的头孢菌素酶)的良好先导化合物,ADC-7是最重要的化合物之一。鲍曼不动杆菌中的抗性决定因素。ADC-7 / S02030配合物的原子结构突显了关键结构决定因素对于识别硼酸的重要性。在本文中,为了阐明在识别R2-羧酸酯(模拟在β-内酰胺中发现的C 3 / C 4)中的作用,我们设计,合成并表征了S02030的六种衍生物(3a)。在这六种化合物中,最好的抑制剂被证明是带有明显负电荷的化合物(化合物3a – c,3h和3j,K i = 44–115 nM),这与衍生物的负电荷相反。诸如酰胺衍生物3d(K i= 224nM)和羟酰胺衍生物3e(K i= 155nM)被省略。为了开发抑制剂在活性位点结合的结构特征,ADC-7与化合物3c,SM23和EC04的复合物的X射线晶体结构被确定。这三种化合物均具有与S02030相同的结构特征,但仅羧基R2侧链不同,从而为探索带负电荷的R2侧链的独特结合方式提供了机会。这种头孢菌素酶在识别方面表现出高度的多功能性,它采用不同的残基直接与羧酸盐相互作用,因此暗示了ADC酶中存在“羧酸盐结合区”而不是结合位点。此外,该类化合物针对鲍曼不动杆菌的耐药临床菌株进行了测试,并与β-内酰胺类抗生素一起有效抑制细菌生长。



























 京公网安备 11010802027423号
京公网安备 11010802027423号