当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Side chain-specific 11/9-helix propensity of α/β-peptides with alternating residue types†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-07 00:00:00 , DOI: 10.1039/c7ob02816d Jaeyeon Lee 1, 2, 3, 4 , Jihyun Shim 1, 2, 3, 4 , Philjae Kang 1, 2, 3, 4 , Moon-Gun Choi 1, 2, 3, 4 , Soo Hyuk Choi 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-07 00:00:00 , DOI: 10.1039/c7ob02816d Jaeyeon Lee 1, 2, 3, 4 , Jihyun Shim 1, 2, 3, 4 , Philjae Kang 1, 2, 3, 4 , Moon-Gun Choi 1, 2, 3, 4 , Soo Hyuk Choi 1, 2, 3, 4
Affiliation
|
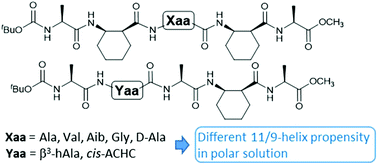
The 11/9-helix is among the most stable and non-traditional helical structures for α/β-peptides with alternating residue types. The effect of side chain groups of α-residues and β3-residues on the 11/9-helix propensity was examined under various solvent conditions. An α-amino acid residue with one of the four representative side chain groups was incorporated into the central position of an α/β-pentapeptide backbone. A β-branched valine residue did not show any destabilizing effect. α,α-Dimethylsubstituted Aib residue was tolerated under nonpolar conditions, but did not promote 11/9-helical folding. The oligomer with a glycine residue did not show 11/9-helical folding under polar solvent conditions. The single unmatched stereochemistry of D-alanine was deleterious to 11/9-helical folding. Replacement of a cyclic β-residue with an acyclic β3-residue in the 11/9-helical structure had a slight destabilizing effect, which could be compensated by a longer peptide sequence with more cyclic β-residues. These results provide a guidance for incorporating functional groups into an 11/9-helical α/β-peptide backbone to design functional oligomers.
中文翻译:

具有交替残基类型的α/β肽的侧链特异性11/9螺旋倾向†
11/9螺旋是具有交替残基类型的α/β肽最稳定且非传统的螺旋结构。α-残基的侧链基团和β的作用3个各种溶剂条件下检查在11/9-螺旋倾向-残基。具有四个代表性侧链基团之一的α-氨基酸残基被并入α/β-五肽主链的中心位置。β-支化缬氨酸残基未显示任何去稳定作用。α,α-二甲基取代的Aib残基在非极性条件下可耐受,但不促进11/9螺旋折叠。具有甘氨酸残基的低聚物在极性溶剂条件下没有显示出11 / 9-螺旋折叠。D的无与伦比的立体化学-丙氨酸对11 / 9-螺旋折叠有害。与环状β残基的置换的非环状β 3个残基在11/9螺旋结构有轻微的去稳定作用,这可能是由较长的肽序列与多个环状β残基来补偿。这些结果为将官能团掺入11 / 9-螺旋α/β-肽主链中以设计功能性低聚物提供了指导。
更新日期:2017-12-07
中文翻译:

具有交替残基类型的α/β肽的侧链特异性11/9螺旋倾向†
11/9螺旋是具有交替残基类型的α/β肽最稳定且非传统的螺旋结构。α-残基的侧链基团和β的作用3个各种溶剂条件下检查在11/9-螺旋倾向-残基。具有四个代表性侧链基团之一的α-氨基酸残基被并入α/β-五肽主链的中心位置。β-支化缬氨酸残基未显示任何去稳定作用。α,α-二甲基取代的Aib残基在非极性条件下可耐受,但不促进11/9螺旋折叠。具有甘氨酸残基的低聚物在极性溶剂条件下没有显示出11 / 9-螺旋折叠。D的无与伦比的立体化学-丙氨酸对11 / 9-螺旋折叠有害。与环状β残基的置换的非环状β 3个残基在11/9螺旋结构有轻微的去稳定作用,这可能是由较长的肽序列与多个环状β残基来补偿。这些结果为将官能团掺入11 / 9-螺旋α/β-肽主链中以设计功能性低聚物提供了指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号