当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use of Phenoxyaniline Analogues To Generate Biochemical Insights into the Interactio n of Polybrominated Diphenyl Ether with CYP2B Enzymes
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.biochem.7b01024 Chao Chen 1 , Jingbao Liu 1 , James R. Halpert 1 , P. Ross Wilderman 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.biochem.7b01024 Chao Chen 1 , Jingbao Liu 1 , James R. Halpert 1 , P. Ross Wilderman 1
Affiliation
|
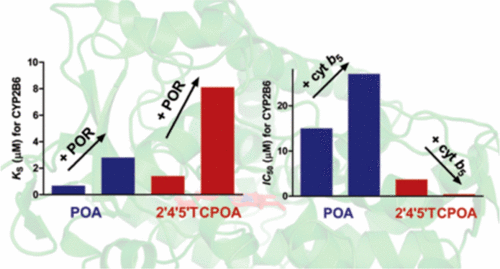
Human hepatic cytochromes P450 (CYP) are integral to xenobiotic metabolism. CYP2B6 is a major catalyst of biotransformation of environmental toxicants, including polybrominated diphenyl ethers (PBDEs). CYP2B substrates tend to contain halogen atoms, but the biochemical basis for this selectivity and for species specific determinants of metabolism has not been identified. Spectral binding titrations and inhibition studies were performed to investigate interactions of rat CYP2B1, rabbit CYP2B4, and CYP2B6 with a series of phenoxyaniline (POA) congeners that are analogues of PBDEs. For most congeners, there was a <3-fold difference between the spectral binding constants (KS) and IC50 values. In contrast, large discrepancies between these values were observed for POA and 3-chloro-4-phenoxyaniline. CYP2B1 was the enzyme most sensitive to POA congeners, so the Val-363 residue from that enzyme was introduced into CYP2B4 or CYP2B6. This substitution partially altered the protein–ligand interaction profiles to make them more similar to that of CYP2B1. Addition of cytochrome P450 oxidoreductase (POR) to titrations of CYP2B6 with POA or 2′4′5′TCPOA decreased the affinity of both ligands for the enzyme. Addition of cytochrome b5 to a recombinant enzyme system containing POR and CYP2B6 increased the POA IC50 value and decreased the 2′4′5′TCPOA IC50 value. Overall, the inconsistency between KS and IC50 values for POA versus 2′4′5′TCPOA is largely due to the effects of redox partner binding. These results provide insight into the biochemical basis of binding of diphenyl ethers to human CYP2B6 and changes in CYP2B6-mediated metabolism that are dependent on POA congener and redox partner identity.
中文翻译:

使用苯氧基苯胺类似物生成多溴联苯醚与CYP2B酶相互作用的生化信息
人肝细胞色素P450(CYP)是异源生物代谢不可或缺的。CYP2B6是环境毒物,包括多溴二苯醚(PBDEs)的生物转化的主要催化剂。CYP2B底物倾向于含有卤素原子,但尚未确定该选择性和物种特异性代谢决定因素的生化基础。进行了光谱结合滴定和抑制研究,以研究大鼠CYP2B1,兔CYP2B4和CYP2B6与一系列与PBDEs类似的苯氧基苯胺(POA)同系物的相互作用。对于大多数同类产品,光谱结合常数(K S)和IC 50之间的差异小于3倍价值观。相反,对于POA和3-氯-4-苯氧基苯胺,观察到这些值之间的巨大差异。CYP2B1是对POA同系物最敏感的酶,因此该酶的Val-363残基被引入CYP2B4或CYP2B6中。这种取代部分改变了蛋白质-配体的相互作用,使其与CYP2B1更相似。在用POA或2'4'5'TCPOA滴定CYP2B6的过程中,添加细胞色素P450氧化还原酶(POR)会降低两个配体对该酶的亲和力。将细胞色素b 5添加到含有POR和CYP2B6的重组酶体系中会增加POA IC 50值,并降低2'4'5'TCPOA IC 50值。总体而言,K S之间的不一致POA与2'4'5'TCPOA的IC 50值很大程度上归因于氧化还原伴侣结合的影响。这些结果提供了对二苯醚与人CYP2B6结合的生物化学基础以及依赖于POA同系物和氧化还原伙伴身份的CYP2B6介导的代谢变化的深入了解。
更新日期:2017-12-19
中文翻译:

使用苯氧基苯胺类似物生成多溴联苯醚与CYP2B酶相互作用的生化信息
人肝细胞色素P450(CYP)是异源生物代谢不可或缺的。CYP2B6是环境毒物,包括多溴二苯醚(PBDEs)的生物转化的主要催化剂。CYP2B底物倾向于含有卤素原子,但尚未确定该选择性和物种特异性代谢决定因素的生化基础。进行了光谱结合滴定和抑制研究,以研究大鼠CYP2B1,兔CYP2B4和CYP2B6与一系列与PBDEs类似的苯氧基苯胺(POA)同系物的相互作用。对于大多数同类产品,光谱结合常数(K S)和IC 50之间的差异小于3倍价值观。相反,对于POA和3-氯-4-苯氧基苯胺,观察到这些值之间的巨大差异。CYP2B1是对POA同系物最敏感的酶,因此该酶的Val-363残基被引入CYP2B4或CYP2B6中。这种取代部分改变了蛋白质-配体的相互作用,使其与CYP2B1更相似。在用POA或2'4'5'TCPOA滴定CYP2B6的过程中,添加细胞色素P450氧化还原酶(POR)会降低两个配体对该酶的亲和力。将细胞色素b 5添加到含有POR和CYP2B6的重组酶体系中会增加POA IC 50值,并降低2'4'5'TCPOA IC 50值。总体而言,K S之间的不一致POA与2'4'5'TCPOA的IC 50值很大程度上归因于氧化还原伴侣结合的影响。这些结果提供了对二苯醚与人CYP2B6结合的生物化学基础以及依赖于POA同系物和氧化还原伙伴身份的CYP2B6介导的代谢变化的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号