当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Binding Kinetics in Multimodal Systems: Implications for Protein Separations
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.analchem.7b04158 Kartik Srinivasan 1 , Mirco Sorci 1 , Lars Sejergaard 1 , Swarnim Ranjan 1 , Georges Belfort 1 , Steven M. Cramer 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.analchem.7b04158 Kartik Srinivasan 1 , Mirco Sorci 1 , Lars Sejergaard 1 , Swarnim Ranjan 1 , Georges Belfort 1 , Steven M. Cramer 1
Affiliation
|
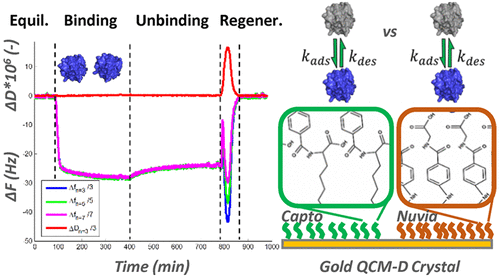
In this work, quartz crystal microbalance with dissipation (QCM-D) was employed to study the kinetic processes involved in the interaction of proteins with self-assembled monolayers (SAMs) of multimodal (MM) ligands. SAMs were fabricated to mimic two chromatographic multimodal resins with varying accessibility of the aromatic moiety to provide a well-defined model system. Kinetic parameters were determined for two different proteins in the presence of the arginine and guanidine and a comparison was made with chromatographic retention data. The results indicated that the accessibility of the ligand’s aromatic moiety can have an important impact on the kinetics and chromatographic retention behavior. Interestingly, arginine and guanidine had very different effects on the protein adsorption and desorption kinetics in these MM systems. For cytochrome C, arginine resulted in a significant decrease and increase in the adsorption and desorption rates, respectively, while guanidine produced a dramatic increase in the desorption rate, with minimal effect on the adsorption rate. In addition, at different concentrations of arginine, two distinct kinetic scenarios were observed. For α-chymotrypsin, the presence of 0.1 M guanidine in the aromatic exposed ligand system produced an increase in the adsorption rate and only a moderate increase in the desorption rate, which helped to explain the surprising increase in the chromatographic salt elution concentration. These results demonstrate that protein adsorption kinetics in the presence of different mobile phase modifiers and MM ligand chemistries can play an important role in contributing to selectivity in MM chromatography.
中文翻译:

多峰系统中的蛋白质结合动力学:蛋白质分离的意义。
在这项工作中,具有耗散的石英晶体微天平(QCM-D)用于研究蛋白质与多峰(MM)配体的自组装单层(SAM)相互作用的动力学过程。制备SAM来模拟两种色谱多峰树脂,芳香族部分的可及性各不相同,以提供定义明确的模型系统。在精氨酸和胍的存在下测定两种不同蛋白质的动力学参数,并与色谱保留数据进行比较。结果表明,配体芳族部分的可及性对动力学和色谱保留行为具有重要影响。有趣的是,精氨酸和胍对这些MM系统中蛋白质的吸附和解吸动力学具有非常不同的影响。对于细胞色素C,精氨酸分别导致吸附和解吸速率显着降低和增加,而胍使解吸速率显着提高,而对吸附速率的影响最小。另外,在不同浓度的精氨酸下,观察到两种不同的动力学情况。对于α-胰凝乳蛋白酶,在芳香族暴露的配体系统中存在0.1 M胍会导致吸附速率增加,而解吸速率仅适度增加,这有助于解释色谱盐洗脱浓度的惊人增加。这些结果表明,在存在不同流动相改性剂和MM配体化学物质的情况下,蛋白质的吸附动力学可以在MM色谱的选择性中发挥重要作用。精氨酸分别导致吸附和解吸速率的显着降低和增加,而胍使解吸速率显着提高,而对吸附速率的影响最小。另外,在不同浓度的精氨酸下,观察到两种不同的动力学情况。对于α-胰凝乳蛋白酶,在芳香族暴露的配体系统中存在0.1 M胍会导致吸附速率增加,而解吸速率仅适度增加,这有助于解释色谱盐洗脱浓度的惊人增加。这些结果表明,在存在不同流动相改性剂和MM配体化学物质的情况下,蛋白质的吸附动力学可以在MM色谱的选择性中发挥重要作用。精氨酸分别导致吸附和解吸速率的显着降低和增加,而胍使解吸速率显着提高,而对吸附速率的影响最小。另外,在不同浓度的精氨酸下,观察到两种不同的动力学情况。对于α-胰凝乳蛋白酶,在芳香族暴露的配体系统中存在0.1 M胍会导致吸附速率增加,而解吸速率仅适度增加,这有助于解释色谱盐洗脱浓度的惊人增加。这些结果表明,在存在不同流动相改性剂和MM配体化学物质的情况下,蛋白质的吸附动力学可以在MM色谱的选择性中发挥重要作用。
更新日期:2018-01-10
中文翻译:

多峰系统中的蛋白质结合动力学:蛋白质分离的意义。
在这项工作中,具有耗散的石英晶体微天平(QCM-D)用于研究蛋白质与多峰(MM)配体的自组装单层(SAM)相互作用的动力学过程。制备SAM来模拟两种色谱多峰树脂,芳香族部分的可及性各不相同,以提供定义明确的模型系统。在精氨酸和胍的存在下测定两种不同蛋白质的动力学参数,并与色谱保留数据进行比较。结果表明,配体芳族部分的可及性对动力学和色谱保留行为具有重要影响。有趣的是,精氨酸和胍对这些MM系统中蛋白质的吸附和解吸动力学具有非常不同的影响。对于细胞色素C,精氨酸分别导致吸附和解吸速率显着降低和增加,而胍使解吸速率显着提高,而对吸附速率的影响最小。另外,在不同浓度的精氨酸下,观察到两种不同的动力学情况。对于α-胰凝乳蛋白酶,在芳香族暴露的配体系统中存在0.1 M胍会导致吸附速率增加,而解吸速率仅适度增加,这有助于解释色谱盐洗脱浓度的惊人增加。这些结果表明,在存在不同流动相改性剂和MM配体化学物质的情况下,蛋白质的吸附动力学可以在MM色谱的选择性中发挥重要作用。精氨酸分别导致吸附和解吸速率的显着降低和增加,而胍使解吸速率显着提高,而对吸附速率的影响最小。另外,在不同浓度的精氨酸下,观察到两种不同的动力学情况。对于α-胰凝乳蛋白酶,在芳香族暴露的配体系统中存在0.1 M胍会导致吸附速率增加,而解吸速率仅适度增加,这有助于解释色谱盐洗脱浓度的惊人增加。这些结果表明,在存在不同流动相改性剂和MM配体化学物质的情况下,蛋白质的吸附动力学可以在MM色谱的选择性中发挥重要作用。精氨酸分别导致吸附和解吸速率的显着降低和增加,而胍使解吸速率显着提高,而对吸附速率的影响最小。另外,在不同浓度的精氨酸下,观察到两种不同的动力学情况。对于α-胰凝乳蛋白酶,在芳香族暴露的配体系统中存在0.1 M胍会导致吸附速率增加,而解吸速率仅适度增加,这有助于解释色谱盐洗脱浓度的惊人增加。这些结果表明,在存在不同流动相改性剂和MM配体化学物质的情况下,蛋白质的吸附动力学可以在MM色谱的选择性中发挥重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号