当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glutathione-Responsive Selenosulfide Prodrugs as a Platform Strategy for Potent and Selective Mechanism-Based Inhibition of Protein Tyrosine Phosphatases
ACS Central Science ( IF 12.7 ) Pub Date : 2017-12-06 00:00:00 , DOI: 10.1021/acscentsci.7b00486 Caroline Chandra Tjin 1 , Kate D. Otley 1 , Tyler D. Baguley 1 , Pradeep Kurup 2 , Jian Xu 2 , Angus C. Nairn 3 , Paul J. Lombroso 2 , Jonathan A. Ellman 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-12-06 00:00:00 , DOI: 10.1021/acscentsci.7b00486 Caroline Chandra Tjin 1 , Kate D. Otley 1 , Tyler D. Baguley 1 , Pradeep Kurup 2 , Jian Xu 2 , Angus C. Nairn 3 , Paul J. Lombroso 2 , Jonathan A. Ellman 1
Affiliation
|
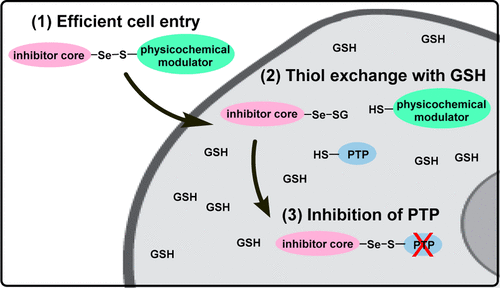
Dysregulation of protein tyrosine phosphorylation has been implicated in a number of human diseases, including cancer, diabetes, and neurodegenerative diseases. As a result of their essential role in regulating protein tyrosine phosphorylation levels, protein tyrosine phosphatases (PTPs) have emerged as important yet challenging therapeutic targets. Here we report on the development and application of a glutathione-responsive motif to facilitate the efficient intracellular delivery of a novel class of selenosulfide phosphatase inhibitors for the selective active site directed inhibition of the targeted PTP by selenosulfide exchange with the active site cysteine. The strategy leverages the large difference in extracellular and intracellular glutathione levels to deliver selenosulfide phosphatase inhibitors to cells. As an initial exploration of the prodrug platform and the corresponding selenosulfide covalent inhibitor class, potent and selective inhibitors were developed for two therapeutically relevant PTP targets: the Mycobacterium tuberculosis virulence factor mPTPA and the CNS-specific tyrosine phosphatase, striatal-enriched protein tyrosine phosphatase (STEP). The lead selenosulfide inhibitors enable potent and selective inhibition of their respective targets over a panel of human PTPs and a representative cysteine protease. Kinetic parameters of the inhibitors were characterized, including reversibility of inhibition and rapid rate of GSH exchange at intracellular GSH concentrations. Additionally, active site covalent inhibitor-labeling with an mPTPA inhibitor was rigorously confirmed by mass spectrometry, and cellular activity was demonstrated with a STEP prodrug inhibitor in cortical neurons.
中文翻译:

谷胱甘肽响应硒化亚砜前药作为基于平台的策略,基于蛋白质酪氨酸磷酸酶的有效和选择性机制抑制。
蛋白酪氨酸磷酸化的失调已经牵涉到许多人类疾病中,包括癌症,糖尿病和神经退行性疾病。由于其在调节蛋白质酪氨酸磷酸化水平中的重要作用,蛋白质酪氨酸磷酸酶(PTP)已成为重要但具有挑战性的治疗靶标。在这里,我们报道了谷胱甘肽响应性基序的发展和应用,以促进新型一类硒代硫化物磷酸酶抑制剂的有效细胞内递送,用于通过与活性位点半胱氨酸的硒代硫化物交换来选择性抑制活性位点。该策略利用细胞外和细胞内谷胱甘肽水平的巨大差异将硒代硫化物磷酸酶抑制剂传递至细胞。结核分枝杆菌毒力因子m PTPA和中枢神经系统特异性酪氨酸磷酸酶,纹状体富集的蛋白质酪氨酸磷酸酶(STEP)。硒代硒化铅抑制剂能够在一组人PTP和代表性的半胱氨酸蛋白酶上有效和选择性地抑制其各自的靶标。表征了抑制剂的动力学参数,包括在细胞内GSH浓度下抑制的可逆性和GSH交换的快速速率。此外,通过质谱严格确认了使用m PTPA抑制剂标记的活性位点共价抑制剂,并用STEP前药抑制剂在皮质神经元中证明了细胞活性。
更新日期:2017-12-06
中文翻译:

谷胱甘肽响应硒化亚砜前药作为基于平台的策略,基于蛋白质酪氨酸磷酸酶的有效和选择性机制抑制。
蛋白酪氨酸磷酸化的失调已经牵涉到许多人类疾病中,包括癌症,糖尿病和神经退行性疾病。由于其在调节蛋白质酪氨酸磷酸化水平中的重要作用,蛋白质酪氨酸磷酸酶(PTP)已成为重要但具有挑战性的治疗靶标。在这里,我们报道了谷胱甘肽响应性基序的发展和应用,以促进新型一类硒代硫化物磷酸酶抑制剂的有效细胞内递送,用于通过与活性位点半胱氨酸的硒代硫化物交换来选择性抑制活性位点。该策略利用细胞外和细胞内谷胱甘肽水平的巨大差异将硒代硫化物磷酸酶抑制剂传递至细胞。结核分枝杆菌毒力因子m PTPA和中枢神经系统特异性酪氨酸磷酸酶,纹状体富集的蛋白质酪氨酸磷酸酶(STEP)。硒代硒化铅抑制剂能够在一组人PTP和代表性的半胱氨酸蛋白酶上有效和选择性地抑制其各自的靶标。表征了抑制剂的动力学参数,包括在细胞内GSH浓度下抑制的可逆性和GSH交换的快速速率。此外,通过质谱严格确认了使用m PTPA抑制剂标记的活性位点共价抑制剂,并用STEP前药抑制剂在皮质神经元中证明了细胞活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号