当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-term G1 cell cycle arrest in cervical cancer cells induced by co-immobilized TNF-α plus IFN-γ polymeric drugs†
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1039/c7tb02608k Wuya Chen 1, 2, 3, 4 , Wenwen Wang 4, 5, 6 , Liyi Chen 1, 2, 3, 4 , Jiamei Chen 1, 2, 3, 4 , Xinhua Lu 1, 2, 3, 4 , Zhibin Li 4, 7, 8, 9, 10 , Baoyan Wu 3, 4, 11, 12, 13 , Liang Yin 1, 2, 3, 4 , Yan-Qing Guan 1, 2, 3, 4, 11
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1039/c7tb02608k Wuya Chen 1, 2, 3, 4 , Wenwen Wang 4, 5, 6 , Liyi Chen 1, 2, 3, 4 , Jiamei Chen 1, 2, 3, 4 , Xinhua Lu 1, 2, 3, 4 , Zhibin Li 4, 7, 8, 9, 10 , Baoyan Wu 3, 4, 11, 12, 13 , Liang Yin 1, 2, 3, 4 , Yan-Qing Guan 1, 2, 3, 4, 11
Affiliation
|
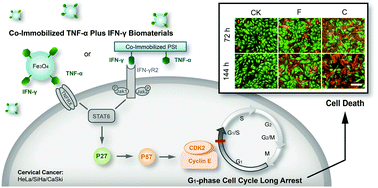
A realistic control of cell cycle arrest is an attractive goal for the development of new effective anti-cancer drugs. Any clinical application of an effective anti-cancer drug necessarily relies on the understanding of cellular interaction mechanisms. In the present study, we prepared a co-immobilized TNF-α plus IFN-γ biomaterial, which showed a significant inhibition effect on cervical cancer cell growth, as demonstrated by a series of structural and cellular characterizations. We found that co-immobilized TNF-α plus IFN-α induced a long-term G1 phase cell cycle arrest in HeLa, SiHa, and CaSki cells, respectively. More surprisingly, the expression level of the p27 protein decreased, even when p27 mRNA was highly expressed. In addition, gene-chip results and microarray analysis showed that p57 may be downstream from p27, which acts as a direct regulator of the long-term G1 cell cycle arrest in these cells, leaving no escape for cervical cancer cells. Finally, we also investigated the anti-tumor mechanism of co-immobilized TNF-α plus IFN-γ in vivo, using a nude mice animal model. To sum up, our findings suggested that the co-immobilized TNF-α plus IFN-γ can induce a long-term cell cycle arrest in cancer, thus serving as a very efficient tool for treating cervical cancer.
中文翻译:

共固定的TNF-α和IFN-γ聚合药物诱导的宫颈癌细胞 的长期G 1细胞周期阻滞†
实际控制细胞周期停滞是开发新的有效抗癌药物的诱人目标。有效的抗癌药的任何临床应用都必须依赖对细胞相互作用机制的理解。在本研究中,我们制备了一种共固定化的TNF-α加IFN-γ生物材料,该材料对宫颈癌细胞的生长具有显着的抑制作用,如一系列结构和细胞特征所示。我们发现,固定化的TNF-α和IFN-α共同诱导了长期的G 1HeLa,SiHa和CaSki细胞中的第三个阶段细胞周期停滞。更令人惊讶的是,即使p27 mRNA高度表达,p27蛋白的表达水平却下降了。此外,基因芯片结果和微阵列分析表明,p57可能位于p27的下游,而p27则是这些细胞中长期G 1细胞周期停滞的直接调节剂,宫颈癌细胞无法逃脱。最后,我们还使用裸鼠动物模型研究了共固定化TNF-α和IFN-γ在体内的抗肿瘤机制。综上所述,我们的研究结果表明,共同固定化的TNF-α和IFN-γ可以诱导癌症的长期细胞周期停滞,从而成为治疗宫颈癌的非常有效的工具。
更新日期:2017-12-05
中文翻译:

共固定的TNF-α和IFN-γ聚合药物诱导的宫颈癌细胞 的长期G 1细胞周期阻滞†
实际控制细胞周期停滞是开发新的有效抗癌药物的诱人目标。有效的抗癌药的任何临床应用都必须依赖对细胞相互作用机制的理解。在本研究中,我们制备了一种共固定化的TNF-α加IFN-γ生物材料,该材料对宫颈癌细胞的生长具有显着的抑制作用,如一系列结构和细胞特征所示。我们发现,固定化的TNF-α和IFN-α共同诱导了长期的G 1HeLa,SiHa和CaSki细胞中的第三个阶段细胞周期停滞。更令人惊讶的是,即使p27 mRNA高度表达,p27蛋白的表达水平却下降了。此外,基因芯片结果和微阵列分析表明,p57可能位于p27的下游,而p27则是这些细胞中长期G 1细胞周期停滞的直接调节剂,宫颈癌细胞无法逃脱。最后,我们还使用裸鼠动物模型研究了共固定化TNF-α和IFN-γ在体内的抗肿瘤机制。综上所述,我们的研究结果表明,共同固定化的TNF-α和IFN-γ可以诱导癌症的长期细胞周期停滞,从而成为治疗宫颈癌的非常有效的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号