当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple Reaction Monitoring for the Quantitation of Serum Protein Glycosylation Profiles: Application to Ovarian Cancer
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.jproteome.7b00541 Suzanne Miyamoto 1 , Carol D. Stroble 1, 2 , Sandra Taylor 3 , Qiuting Hong 2 , Carlito B. Lebrilla 2 , Gary S. Leiserowitz 4 , Kyoungmi Kim 3 , L. Renee Ruhaak 2, 5
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.jproteome.7b00541 Suzanne Miyamoto 1 , Carol D. Stroble 1, 2 , Sandra Taylor 3 , Qiuting Hong 2 , Carlito B. Lebrilla 2 , Gary S. Leiserowitz 4 , Kyoungmi Kim 3 , L. Renee Ruhaak 2, 5
Affiliation
|
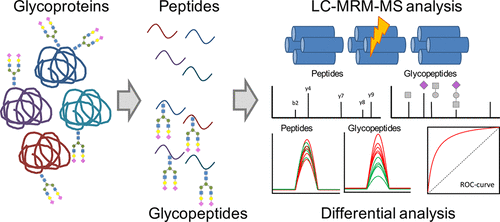
Protein glycosylation fingerprints are widely recognized as potential markers for disease states, and indeed differential glycosylation has been identified in multiple types of autoimmune diseases and several types of cancer. However, releasing the glycans leave the glycoproteins unknown; therefore, there exists a need for high-throughput methods that allow quantification of site- and protein-specific glycosylation patterns from complex biological mixtures. In this study, a targeted multiple reaction monitoring (MRM)-based method for the protein- and site-specific quantitation involving serum proteins immunoglobulins A, G and M, alpha-1-antitrypsin, transferrin, alpha-2-macroglobulin, haptoglobin, alpha-1-acid glycoprotein and complement C3 was developed. The method is based on tryptic digestion of serum glycoproteins, followed by immediate reverse phase UPLC-QQQ-MS analysis of glycopeptides. To quantitate protein glycosylation independent of the protein serum concentration, a nonglycosylated peptide was also monitored. Using this strategy, 178 glycopeptides and 18 peptides from serum glycoproteins are analyzed with good repeatability (interday CVs of 3.65–21–92%) in a single 17 min run. To assess the potential of the method, protein glycosylation was analyzed in serum samples from ovarian cancer patients and controls. A training set consisting of 40 cases and 40 controls was analyzed, and differential analyses were performed to identify aberrant glycopeptide levels. All findings were validated in an independent test set (n = 44 cases and n = 44 controls). In addition to the differential glycosylation on the immunoglobulins, which was reported previously, aberrant glycosylation was also observed on each of the glycoproteins, which could be corroborated in the test set. This report shows the development of a method for targeted protein- and site-specific glycosylation analysis and the potential of such methods in biomarker development.
中文翻译:

血清蛋白糖基化谱定量的多反应监测:在卵巢癌中的应用
蛋白质糖基化指纹被广泛认为是疾病状态的潜在标志物,事实上,在多种类型的自身免疫性疾病和几种癌症中已经鉴定出差异糖基化。然而,释放聚糖使糖蛋白未知。因此,需要一种高通量的方法,该方法允许定量来自复杂生物混合物的位点和蛋白质特异性糖基化模式。在这项研究中,针对蛋白质和位点的定量分析采用了基于靶向多反应监测(MRM)的方法,涉及血清蛋白免疫球蛋白A,G和M,α-1-抗胰蛋白酶,转铁蛋白,α-2-巨球蛋白,触珠蛋白,开发了α-1-酸糖蛋白和补体C3。该方法基于血清糖蛋白的胰蛋白酶消化,然后立即进行糖肽的反相UPLC-QQQ-MS分析。为了定量独立于蛋白质血清浓度的蛋白质糖基化,还监测了非糖基化的肽。使用此策略,可以在17分钟内分析血清糖蛋白中的178个糖肽和18个肽,并具有良好的重复性(日CV为3.65–21–92%)。为了评估该方法的潜力,对来自卵巢癌患者和对照的血清样品中的蛋白质糖基化进行了分析。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(还监测了非糖基化的肽。使用此策略,可以在17分钟内分析血清糖蛋白中的178个糖肽和18个肽,并具有良好的重复性(日CV为3.65–21–92%)。为了评估该方法的潜力,对来自卵巢癌患者和对照的血清样品中的蛋白质糖基化进行了分析。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(还监测了非糖基化的肽。使用此策略,可以在17分钟内分析血清糖蛋白中的178个糖肽和18个肽,并具有良好的重复性(日CV为3.65–21–92%)。为了评估该方法的潜力,对来自卵巢癌患者和对照的血清样品中的蛋白质糖基化进行了分析。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(在卵巢癌患者和对照的血清样本中分析了蛋白质糖基化。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(在卵巢癌患者和对照的血清样本中分析了蛋白质糖基化。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(n = 44例,n = 44个对照)。除了先前报道的免疫球蛋白上的差异糖基化以外,还在每种糖蛋白上观察到异常的糖基化,这可以在测试集中得到证实。该报告显示了针对目标蛋白和位点特异性糖基化分析方法的开发以及此类方法在生物标记开发中的潜力。
更新日期:2017-12-19
中文翻译:

血清蛋白糖基化谱定量的多反应监测:在卵巢癌中的应用
蛋白质糖基化指纹被广泛认为是疾病状态的潜在标志物,事实上,在多种类型的自身免疫性疾病和几种癌症中已经鉴定出差异糖基化。然而,释放聚糖使糖蛋白未知。因此,需要一种高通量的方法,该方法允许定量来自复杂生物混合物的位点和蛋白质特异性糖基化模式。在这项研究中,针对蛋白质和位点的定量分析采用了基于靶向多反应监测(MRM)的方法,涉及血清蛋白免疫球蛋白A,G和M,α-1-抗胰蛋白酶,转铁蛋白,α-2-巨球蛋白,触珠蛋白,开发了α-1-酸糖蛋白和补体C3。该方法基于血清糖蛋白的胰蛋白酶消化,然后立即进行糖肽的反相UPLC-QQQ-MS分析。为了定量独立于蛋白质血清浓度的蛋白质糖基化,还监测了非糖基化的肽。使用此策略,可以在17分钟内分析血清糖蛋白中的178个糖肽和18个肽,并具有良好的重复性(日CV为3.65–21–92%)。为了评估该方法的潜力,对来自卵巢癌患者和对照的血清样品中的蛋白质糖基化进行了分析。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(还监测了非糖基化的肽。使用此策略,可以在17分钟内分析血清糖蛋白中的178个糖肽和18个肽,并具有良好的重复性(日CV为3.65–21–92%)。为了评估该方法的潜力,对来自卵巢癌患者和对照的血清样品中的蛋白质糖基化进行了分析。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(还监测了非糖基化的肽。使用此策略,可以在17分钟内分析血清糖蛋白中的178个糖肽和18个肽,并具有良好的重复性(日CV为3.65–21–92%)。为了评估该方法的潜力,对来自卵巢癌患者和对照的血清样品中的蛋白质糖基化进行了分析。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(在卵巢癌患者和对照的血清样本中分析了蛋白质糖基化。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(在卵巢癌患者和对照的血清样本中分析了蛋白质糖基化。分析了由40例病例和40例对照组成的训练集,并进行了差异分析以识别异常的糖肽水平。所有发现均在独立的测试集中进行了验证(n = 44例,n = 44个对照)。除了先前报道的免疫球蛋白上的差异糖基化以外,还在每种糖蛋白上观察到异常的糖基化,这可以在测试集中得到证实。该报告显示了针对目标蛋白和位点特异性糖基化分析方法的开发以及此类方法在生物标记开发中的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号