当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Separation Behavior of Supercharged Proteins and Polyelectrolytes
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.biochem.7b00990 Chad S. Cummings 1 , Allie C. Obermeyer 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.biochem.7b00990 Chad S. Cummings 1 , Allie C. Obermeyer 1
Affiliation
|
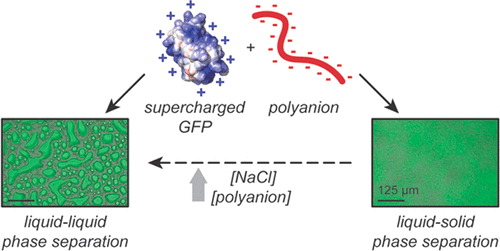
Membraneless organelles, like membrane-bound organelles, are essential to cell homeostasis and provide discrete cellular subcompartments. Unlike classical organelles, membraneless organelles possess no physical barrier but rather arise by phase separation of the organelle components from the surrounding cytoplasm or nucleoplasm. Complex coacervation, the liquid–liquid phase separation of oppositely charged polyelectrolytes, is one of several phenomena that are hypothesized to drive the formation and regulation of some membraneless organelles. Studies of the molecular properties of globular proteins that drive complex coacervation are limited as many proteins do not form complexes with oppositely charged macromolecules at neutral pH and moderate ionic strengths. Protein supercharging overcomes this problem and drives complexation with oppositely charged macromolecules. In this work, several distinct cationic supercharged green fluorescent protein (GFP) variants were designed to examine the phase behavior with oppositely charged polyanionic macromolecules. Cationic GFP variants phase separated with oppositely charged macromolecules at various mixing ratios, salt concentrations, and pH values. Efficient protein incorporation in the macromolecule rich phase occurred over a range of protein and polymer mass fractions, but the protein encapsulation efficiency was highest at the midpoint of the phase separation regime. More positively charged proteins phase separated over broader pH and salt ranges than those of proteins with a lower charge density. Interestingly, each GFP variant phase separated at higher salt concentrations with anionic synthetic macromolecules compared to anionic biological macromolecules. Optical microscopy revealed that most variants, depending on solution conditions, formed liquid–liquid phase separations, except for GFP/DNA pairs that formed solid aggregates under all tested conditions.
中文翻译:

增压的蛋白质和聚电解质的相分离行为
像膜结合细胞器一样,无膜细胞器对于细胞稳态是必不可少的,并提供离散的细胞亚区室。与经典细胞器不同,无膜细胞器没有物理屏障,而是通过细胞器组分与周围细胞质或核质的相分离而产生的。复杂的凝聚,即带相反电荷的聚电解质的液-液相分离,是被认为驱动某些无膜细胞器形成和调节的几种现象之一。由于许多蛋白质在中性pH和中等离子强度下不会与带相反电荷的大分子形成复合物,因此对驱动复合物凝聚的球形蛋白质分子特性的研究受到限制。蛋白质增压克服了这个问题,并推动了带相反电荷的大分子的络合。在这项工作中,设计了几种不同的阳离子带电绿色荧光蛋白(GFP)变体,以检查带相反电荷的聚阴离子大分子的相行为。阳离子GFP变体在各种混合比,盐浓度和pH值下与带相反电荷的大分子相分离。在丰富的蛋白质和聚合物质量分数范围内,有效的蛋白质掺入大分子富集相发生了,但是蛋白质的包封效率在相分离方案的中点最高。与具有较低电荷密度的蛋白质相比,在更宽的pH和盐范围内,带正电荷的蛋白质会发生相分离。有趣的是,与阴离子生物大分子相比,每个GFP变体相都以较高的盐浓度与阴离子合成大分子分离。光学显微镜显示,除了GFP / DNA对在所有测试条件下均形成固体聚集体外,大多数变体均根据溶液条件而形成液-液相分离。
更新日期:2017-12-28
中文翻译:

增压的蛋白质和聚电解质的相分离行为
像膜结合细胞器一样,无膜细胞器对于细胞稳态是必不可少的,并提供离散的细胞亚区室。与经典细胞器不同,无膜细胞器没有物理屏障,而是通过细胞器组分与周围细胞质或核质的相分离而产生的。复杂的凝聚,即带相反电荷的聚电解质的液-液相分离,是被认为驱动某些无膜细胞器形成和调节的几种现象之一。由于许多蛋白质在中性pH和中等离子强度下不会与带相反电荷的大分子形成复合物,因此对驱动复合物凝聚的球形蛋白质分子特性的研究受到限制。蛋白质增压克服了这个问题,并推动了带相反电荷的大分子的络合。在这项工作中,设计了几种不同的阳离子带电绿色荧光蛋白(GFP)变体,以检查带相反电荷的聚阴离子大分子的相行为。阳离子GFP变体在各种混合比,盐浓度和pH值下与带相反电荷的大分子相分离。在丰富的蛋白质和聚合物质量分数范围内,有效的蛋白质掺入大分子富集相发生了,但是蛋白质的包封效率在相分离方案的中点最高。与具有较低电荷密度的蛋白质相比,在更宽的pH和盐范围内,带正电荷的蛋白质会发生相分离。有趣的是,与阴离子生物大分子相比,每个GFP变体相都以较高的盐浓度与阴离子合成大分子分离。光学显微镜显示,除了GFP / DNA对在所有测试条件下均形成固体聚集体外,大多数变体均根据溶液条件而形成液-液相分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号