当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights on Active Sites of CaAl-Hydrotalcite as a High-Performance Solid Base Catalyst toward Aldol Condensation
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acscatal.7b03022 Weihan Bing 1 , Lei Zheng 2 , Shan He 1 , Deming Rao 1 , Ming Xu 1 , Lirong Zheng 2 , Bin Wang 3 , Yangdong Wang 4 , Min Wei 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acscatal.7b03022 Weihan Bing 1 , Lei Zheng 2 , Shan He 1 , Deming Rao 1 , Ming Xu 1 , Lirong Zheng 2 , Bin Wang 3 , Yangdong Wang 4 , Min Wei 1
Affiliation
|
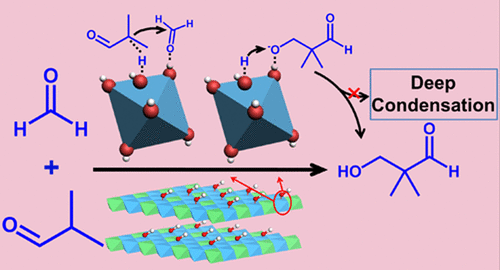
Solid base catalysts are highly demanded in many heterogeneous catalysis processes, but insights on active sites and structure–property correlation have rarely been revealed and remain a challenge. Herein, activated MxAl-layered double hydroxides (denoted as re-MxAl-LDH, M = Ca, Mg), as solid base catalysts toward the aldol condensation reaction, were prepared via a two-step procedure: calcination of the MxAl-LDH precursor to produce MxAl-mixed metal oxide (MxAl-MMO), followed by a further rehydration treatment. Structural characterizations confirm that re-CaxAl-LDH and re-MgxAl-LDH show similar morphology, particle size, specific surface area, and pore size distribution. However, a combination study including XPS, EXAFS, CDCl3–FTIR, and DFT calculations verifies that the host layer of re-CaxAl-LDH contains a distorted Ca(OH)6 octahedron with an additional Ca–OH coordination that provides weak Brønsted basic site; in contrast, re-MgxAl-LDH consists of a Mg(OH)6 octahedron structure accompanied by surface adsorbed hydroxyl group via noncovalent interaction, resulting in a medium Brønsted basic site. For the re-CaxAl-LDH samples, the concentration of weak Brønsted basic sites can be enhanced by tuning the Ca/Al molar ratio in the LDH precursor. The optimized re-CaxAl-LDH catalyst (re-Ca4Al-LDH) exhibits prominent catalytic performance toward the condensation of isobutyraldehyde (IBD) and formaldehyde (FA) to produce hydroxypivaldehyde (HPA), with a HPA yield of 61.5%. This is much higher than that of re-MgxAl-LDH (12.2%) and conventional solid base catalysts (4–33%), and even comparable to the level of liquid alkali (55–72%). Studies on the structure–property correlation reveal that the weak Brønsted basic site serves as active center to catalyze the aldol condensation, which accelerates the product desorption and largely promotes the HPA selectivity.
中文翻译:

CaAl水滑石作为高性能固体碱催化剂对Aldol缩合反应的活性位点的见解
在许多非均相催化过程中对固体碱催化剂都有很高的要求,但是关于活性位点和结构-性质相关性的见解很少被揭示,并且仍然是一个挑战。在此,通过两步程序制备了活化的M x Al层状双氢氧化物(表示为re-M x Al-LDH,M = Ca,Mg),作为用于醇醛缩合反应的固体碱催化剂。 M x Al-LDH前体,以生产M x Al混合金属氧化物(M x Al-MMO),然后进行进一步的水化处理。结构表征证实了re-Ca x Al-LDH和re-Mg xAl-LDH表现出相似的形态,粒度,比表面积和孔径分布。但是,包括XPS,EXAFS,CDCl 3 -FTIR和DFT计算在内的组合研究验证了re-Ca x Al-LDH的主体层包含扭曲的Ca(OH)6八面体,并且具有额外的Ca-OH配位,可提供弱的Brønsted基本站点;相反,re-Mg x Al-LDH由Mg(OH)6八面体结构组成,并通过非共价相互作用伴随表面吸附的羟基,形成中等的布朗斯台德碱性位。对于重新Ca x在Al-LDH样品中,可以通过调整LDH前体中的Ca / Al摩尔比来提高弱Brønsted碱性位点的浓度。优化的re-Ca x Al-LDH催化剂(re-Ca 4 Al-LDH)对异丁醛(IBD)和甲醛(FA)缩合生成羟基戊醛(HPA)表现出突出的催化性能,HPA产率为61.5% 。这远高于再镁x Al-LDH(12.2%)和常规固体碱催化剂(4-33%)的水平,甚至与液态碱金属的水平(55-72%)相当。对结构-性质相关性的研究表明,弱的布朗斯台德碱性位点是催化醛醇缩合的活性中心,这加速了产物的解吸并大大提高了HPA的选择性。
更新日期:2017-12-20
中文翻译:

CaAl水滑石作为高性能固体碱催化剂对Aldol缩合反应的活性位点的见解
在许多非均相催化过程中对固体碱催化剂都有很高的要求,但是关于活性位点和结构-性质相关性的见解很少被揭示,并且仍然是一个挑战。在此,通过两步程序制备了活化的M x Al层状双氢氧化物(表示为re-M x Al-LDH,M = Ca,Mg),作为用于醇醛缩合反应的固体碱催化剂。 M x Al-LDH前体,以生产M x Al混合金属氧化物(M x Al-MMO),然后进行进一步的水化处理。结构表征证实了re-Ca x Al-LDH和re-Mg xAl-LDH表现出相似的形态,粒度,比表面积和孔径分布。但是,包括XPS,EXAFS,CDCl 3 -FTIR和DFT计算在内的组合研究验证了re-Ca x Al-LDH的主体层包含扭曲的Ca(OH)6八面体,并且具有额外的Ca-OH配位,可提供弱的Brønsted基本站点;相反,re-Mg x Al-LDH由Mg(OH)6八面体结构组成,并通过非共价相互作用伴随表面吸附的羟基,形成中等的布朗斯台德碱性位。对于重新Ca x在Al-LDH样品中,可以通过调整LDH前体中的Ca / Al摩尔比来提高弱Brønsted碱性位点的浓度。优化的re-Ca x Al-LDH催化剂(re-Ca 4 Al-LDH)对异丁醛(IBD)和甲醛(FA)缩合生成羟基戊醛(HPA)表现出突出的催化性能,HPA产率为61.5% 。这远高于再镁x Al-LDH(12.2%)和常规固体碱催化剂(4-33%)的水平,甚至与液态碱金属的水平(55-72%)相当。对结构-性质相关性的研究表明,弱的布朗斯台德碱性位点是催化醛醇缩合的活性中心,这加速了产物的解吸并大大提高了HPA的选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号