当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modeling of Human Fatty Acid Synthase and in Silico Docking of Acyl Carrier Protein Domain and Its Partner Catalytic Domains
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.jpcb.7b09645 Matilde F. Viegas 1 , Rui P. P. Neves 1 , Maria J. Ramos 1 , Pedro A. Fernandes 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.jpcb.7b09645 Matilde F. Viegas 1 , Rui P. P. Neves 1 , Maria J. Ramos 1 , Pedro A. Fernandes 1
Affiliation
|
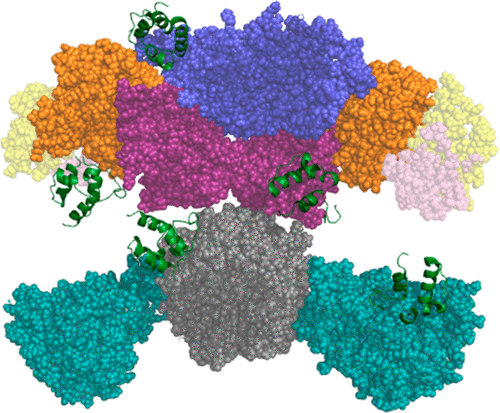
Human fatty acid synthase (hFAS) is a megasynthase whose main function is de novo biosynthesis of saturated fatty acids. Interest has been drawn to this enzyme beyond its physiological role due to the association between high levels of hFAS and clinical conditions such as obesity, diabetes, and cancer. Thus, it has become an undeniably attractive pharmacological target. Until now, no crystal structure of the complete hFAS is available, hindering attempts to fully understand this protein. Using homology modeling, we built a model of the entire megasynthase, encompassing all of its domains, including the acyl carrier protein (ACP) and thioesterase (TE) mobile domains absent in the crystal structure of mammalian fatty acid synthase (FAS). On a second stage, we used data-driven protein–protein docking between the substrate shuttling domain ACP and every catalytic domain in the protein. We also propose sets of amino acids at the interface of each domain that we believe are important to favor the interaction between ACP and each domain of hFAS. After inspection, we validated each complex between ACP and MAT/KS/KR/DH/ER domains through classical molecular dynamics simulations and RMSd analysis. Additionally, we mapped the interactions between the residues at the active site of each catalytic domain and its intermediaries. In every docking, we ensured that the distance between catalytic residues and the intermediaries was maintained. Until now, there was not a complete 3D model of this megasynthase. This study is the first to present a homology model for the whole hFAS, including its two mobile domains and possible poses of ACP throughout the cycle of fatty acid biosynthesis, thus mapping obligatory checkpoints in its trajectory. Hence, we believe that these structural insights will allow for future studies of the catalytic mechanism of the overall hFAS.
中文翻译:

人脂肪酸合成酶的建模和酰基载体蛋白结构域及其伙伴催化结构域的计算机对接
人脂肪酸合成酶(hFAS)是一种巨合成酶,其主要功能是从头开始饱和脂肪酸的生物合成。由于高水平的hFAS与肥胖,糖尿病和癌症等临床状况之间的联系,人们对该酶的兴趣已超出其生理作用。因此,它已成为不可否认的有吸引力的药理靶标。到目前为止,尚无完整hFAS的晶体结构可用,这妨碍了人们全面了解这种蛋白质的尝试。使用同源性建模,我们建立了一个完整的合成酶模型,涵盖了其所有域,包括哺乳动物脂肪酸合成酶(FAS)的晶体结构中不存在的酰基载体蛋白(ACP)和硫酯酶(TE)移动域。在第二阶段,我们使用了数据驱动的蛋白质-蛋白质穿梭在底物穿梭域ACP和蛋白质中的每个催化域之间。我们还提出了每个域的界面上的氨基酸集,我们认为这些氨基酸对促进ACP和hFAS的每个域之间的相互作用很重要。经过检查,我们通过经典的分子动力学模拟和RMSd分析验证了ACP和MAT / KS / KR / DH / ER域之间的每个复合物。此外,我们绘制了每个催化域的活性位点上的残基与其中介之间的相互作用。在每个对接中,我们确保催化残基与中间体之间的距离保持不变。到现在为止,还没有完整的3D模型。这项研究首次提出了整个hFAS的同源性模型,包括其两个可移动域和整个脂肪酸生物合成周期中ACP的可能姿势,从而在其轨迹中绘制了强制性检查点。
更新日期:2018-01-02
中文翻译:

人脂肪酸合成酶的建模和酰基载体蛋白结构域及其伙伴催化结构域的计算机对接
人脂肪酸合成酶(hFAS)是一种巨合成酶,其主要功能是从头开始饱和脂肪酸的生物合成。由于高水平的hFAS与肥胖,糖尿病和癌症等临床状况之间的联系,人们对该酶的兴趣已超出其生理作用。因此,它已成为不可否认的有吸引力的药理靶标。到目前为止,尚无完整hFAS的晶体结构可用,这妨碍了人们全面了解这种蛋白质的尝试。使用同源性建模,我们建立了一个完整的合成酶模型,涵盖了其所有域,包括哺乳动物脂肪酸合成酶(FAS)的晶体结构中不存在的酰基载体蛋白(ACP)和硫酯酶(TE)移动域。在第二阶段,我们使用了数据驱动的蛋白质-蛋白质穿梭在底物穿梭域ACP和蛋白质中的每个催化域之间。我们还提出了每个域的界面上的氨基酸集,我们认为这些氨基酸对促进ACP和hFAS的每个域之间的相互作用很重要。经过检查,我们通过经典的分子动力学模拟和RMSd分析验证了ACP和MAT / KS / KR / DH / ER域之间的每个复合物。此外,我们绘制了每个催化域的活性位点上的残基与其中介之间的相互作用。在每个对接中,我们确保催化残基与中间体之间的距离保持不变。到现在为止,还没有完整的3D模型。这项研究首次提出了整个hFAS的同源性模型,包括其两个可移动域和整个脂肪酸生物合成周期中ACP的可能姿势,从而在其轨迹中绘制了强制性检查点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号