当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A pH and salt dually responsive emulsion in the presence of amphiphilic macromolecules†
Soft Matter ( IF 2.9 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1039/c7sm01760j Guangxin Hu 1, 2, 3, 4 , Hui Yang 5, 6, 7, 8, 9 , Qingfeng Hou 4, 10, 11, 12, 13 , Donghong Guo 4, 10, 11, 12, 13 , Gang Chen 5, 6, 7, 8, 9 , Fanghui Liu 5, 6, 7, 8, 9 , Ting Chen 5, 6, 7, 8, 9 , Xuefeng Shi 5, 6, 7, 8, 9 , Yu Su 1, 2, 3, 4 , Jinben Wang 5, 6, 7, 8, 9
Soft Matter ( IF 2.9 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1039/c7sm01760j Guangxin Hu 1, 2, 3, 4 , Hui Yang 5, 6, 7, 8, 9 , Qingfeng Hou 4, 10, 11, 12, 13 , Donghong Guo 4, 10, 11, 12, 13 , Gang Chen 5, 6, 7, 8, 9 , Fanghui Liu 5, 6, 7, 8, 9 , Ting Chen 5, 6, 7, 8, 9 , Xuefeng Shi 5, 6, 7, 8, 9 , Yu Su 1, 2, 3, 4 , Jinben Wang 5, 6, 7, 8, 9
Affiliation
|
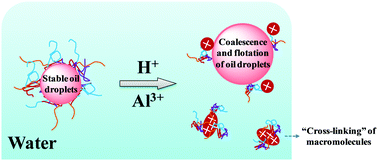
A pH and salt dually responsive emulsion has been designed on the basis of a novel amphiphilic macromolecule. It was found that the water separation of an oil-in-water emulsion reached up to ∼60% after standing for 10 min at low pH. 2-(Diethylamino)ethyl methacrylate (DEA) residues were found to induce the macromolecules to protonate and to be hydrophilic at pH values between 2 and 6, resulting in dewetting from oil droplet surfaces in water. Besides, the macromolecules form aggregates with different structures at the water/oil interface, depending on the pH value or salt concentration of the emulsion system, enabling the system to be demulsified in response to the pH or salt stimulus. The experimental results also showed that with the addition of aluminium chloride at 100 mg L−1, the water separation was about 70% after 20 min. A possible mechanism with respect to demulsifying was proposed on the basis of an “ion bridge” among sodium acrylate (SA) residues, inducing the macromolecules to “cross-link” and become insoluble, and leading to oil/water separation. Furthermore, at a fixed pH of 5, addition of salt to the aqueous dispersion increased the degree of oil–water interfacial activity and batch emulsions were significantly unstable to coalesce at a low salinity of 25–50 mg L−1. This finding presents a new manipulation on emulsion stability and potential applications in the fields of oil recovery, wastewater treatment, sludge removal, and so on.
中文翻译:

两亲大分子存在下的pH和盐双反应型乳液†
已基于新型两亲大分子设计了pH和盐双反应型乳液。发现在低pH下静置10分钟后,水包油型乳液的水分离率高达〜60%。发现甲基丙烯酸2-(二乙氨基)乙酯(DEA)残留物可诱导大分子质子化,并在pH值为2至6之间具有亲水性,从而导致从水中的油滴表面脱湿。此外,取决于乳液体系的pH值或盐浓度,大分子在水/油界面处形成具有不同结构的聚集体,从而使体系能够响应于pH或盐刺激而破乳。实验结果还表明,添加100 mg L -1的氯化铝20分钟后,水分离率约为70%。在丙烯酸钠(SA)残基之间存在“离子桥”的基础上,提出了一种可能的破乳机理,该分子导致大分子“交联”并变得不溶,并导致油/水分离。此外,在5的固定pH下,向水分散液中添加盐会增加油水界面活性的程度,并且在低盐度(25–50 mg L -1)下,间歇乳液显着不稳定,难以聚结。这一发现提出了一种关于乳状液稳定性的新方法,以及在采油,废水处理,污泥去除等领域的潜在应用。
更新日期:2017-12-05
中文翻译:

两亲大分子存在下的pH和盐双反应型乳液†
已基于新型两亲大分子设计了pH和盐双反应型乳液。发现在低pH下静置10分钟后,水包油型乳液的水分离率高达〜60%。发现甲基丙烯酸2-(二乙氨基)乙酯(DEA)残留物可诱导大分子质子化,并在pH值为2至6之间具有亲水性,从而导致从水中的油滴表面脱湿。此外,取决于乳液体系的pH值或盐浓度,大分子在水/油界面处形成具有不同结构的聚集体,从而使体系能够响应于pH或盐刺激而破乳。实验结果还表明,添加100 mg L -1的氯化铝20分钟后,水分离率约为70%。在丙烯酸钠(SA)残基之间存在“离子桥”的基础上,提出了一种可能的破乳机理,该分子导致大分子“交联”并变得不溶,并导致油/水分离。此外,在5的固定pH下,向水分散液中添加盐会增加油水界面活性的程度,并且在低盐度(25–50 mg L -1)下,间歇乳液显着不稳定,难以聚结。这一发现提出了一种关于乳状液稳定性的新方法,以及在采油,废水处理,污泥去除等领域的潜在应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号