当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical and Physical Variability in Structural Isomers of an l/d α-Sheet Peptide Designed To Inhibit Amyloidogenesis
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.biochem.7b00345 Nathan L. Maris 1 , Dylan Shea 1 , Alissa Bleem 1 , James D. Bryers 1 , Valerie Daggett 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.biochem.7b00345 Nathan L. Maris 1 , Dylan Shea 1 , Alissa Bleem 1 , James D. Bryers 1 , Valerie Daggett 1
Affiliation
|
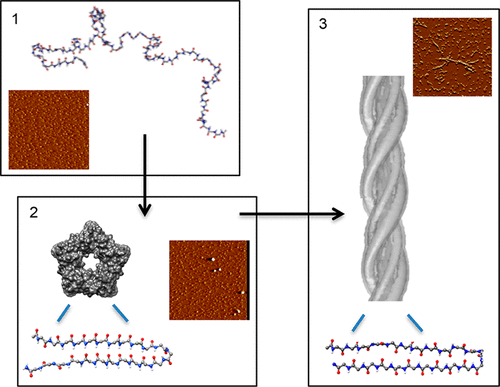
There has been much interest in synthetic peptides as inhibitors of aggregation associated with amyloid diseases. Of particular interest are compounds that target the cytotoxic soluble oligomers preceding the formation of mature, nontoxic fibrils. This study explores physical and chemical differences between two de novo-designed peptides that share an identical primary structure but differ in backbone chirality at six key positions. We show that the presence of alternating l/d-amino acid motifs dramatically increases aqueous solubility, enforces α-sheet secondary structure, and inhibits aggregation of the β-amyloid peptide implicated in Alzheimer’s disease, in addition to neutralizing its cytotoxicity. In contrast, the all-l-amino acid isomer does not form α-sheet structure and is insoluble and inactive.
中文翻译:

抑制淀粉样蛋白形成的1 / dα-Sheet肽结构异构体的化学和物理变异性
合成肽作为与淀粉样蛋白疾病相关的聚集的抑制剂已经引起了很多兴趣。特别感兴趣的是在形成成熟的,无毒的原纤维之前靶向细胞毒性的可溶性低聚物的化合物。这项研究探索了两个从头设计的肽之间的物理和化学差异,这些肽具有相同的一级结构,但在六个关键位置的骨架手性不同。我们表明,交替的l / d-氨基酸基序的存在除中和其细胞毒性外,还显着增加了水溶性,增强了α-折叠二级结构,并抑制了与阿尔茨海默氏病有关的β-淀粉样肽的聚集。相比之下,清一色升-氨基酸异构体不形成α-片层结构,并且是不溶的和不活泼的。
更新日期:2017-12-19
中文翻译:

抑制淀粉样蛋白形成的1 / dα-Sheet肽结构异构体的化学和物理变异性
合成肽作为与淀粉样蛋白疾病相关的聚集的抑制剂已经引起了很多兴趣。特别感兴趣的是在形成成熟的,无毒的原纤维之前靶向细胞毒性的可溶性低聚物的化合物。这项研究探索了两个从头设计的肽之间的物理和化学差异,这些肽具有相同的一级结构,但在六个关键位置的骨架手性不同。我们表明,交替的l / d-氨基酸基序的存在除中和其细胞毒性外,还显着增加了水溶性,增强了α-折叠二级结构,并抑制了与阿尔茨海默氏病有关的β-淀粉样肽的聚集。相比之下,清一色升-氨基酸异构体不形成α-片层结构,并且是不溶的和不活泼的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号