当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
O-GalNAcylation of RANTES Improves Its Properties as a Human Immunodeficiency Virus Type 1 Entry Inhibitor
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00875 Xiaoyang Guan 1 , Patrick K. Chaffey 1 , Huan Chen 2 , Wei Feng 3 , Xiuli Wei 4 , Liu-Meng Yang 2 , Yuan Ruan 1 , Xinfeng Wang 1 , Yaohao Li 1 , Kimberly B. Barosh 1 , Amy H. Tran 1 , Jaimie Zhu 1 , Wei Liang 4 , Yong-Tang Zheng 2 , Xu Wang 3 , Zhongping Tan 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00875 Xiaoyang Guan 1 , Patrick K. Chaffey 1 , Huan Chen 2 , Wei Feng 3 , Xiuli Wei 4 , Liu-Meng Yang 2 , Yuan Ruan 1 , Xinfeng Wang 1 , Yaohao Li 1 , Kimberly B. Barosh 1 , Amy H. Tran 1 , Jaimie Zhu 1 , Wei Liang 4 , Yong-Tang Zheng 2 , Xu Wang 3 , Zhongping Tan 1
Affiliation
|
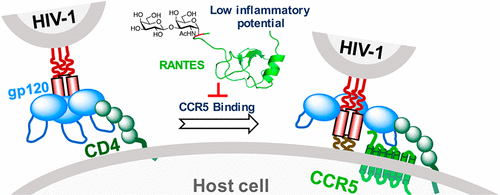
Many human proteins have the potential to be developed as therapeutic agents. However, side effects caused by direct administration of natural proteins have significantly slowed expansion of protein therapeutics into the clinic. Post-translational modifications (PTMs) can improve protein properties, but because of significant knowledge gaps, we are considerably limited in our ability to apply PTMs to generate better protein therapeutics. Here, we seek to fill the gaps by studying the PTMs of a small representative chemotactic cytokine, RANTES. RANTES can inhibit HIV-1 infection by competing with it for binding to receptor CCR5 and stimulating CCR5 endocytosis. Unfortunately, RANTES can induce strong signaling, leading to severe inflammatory side effects. We apply a chemical biology approach to explore the potential of post-translationally modified RANTES as safe inhibitors of HIV-1 infection. We synthesized and systematically tested a library of RANTES isoforms for their ability to inhibit inflammatory signaling and prevent HIV-1 infection of primary human cells. Through this research, we revealed that most of the glycosylated variants have decreased inflammation-associated properties and identified one particular glyco variant, a truncated RANTES containing a Galβ1–3GalNAc disaccharide α-linked to Ser4, which stands out as having the best overall properties: relatively high HIV-1 inhibition potency but also weak inflammatory properties. Moreover, our results provided a structural basis for the observed changes in the properties of RANTES. Taken together, this work highlights the potential importance of glycosylation as an alternative strategy for developing CCR5 inhibitors to treat HIV-1 infection and, more generally, for reducing or eliminating unwanted properties of therapeutic proteins.
中文翻译:

RANTES的O-GalNAcylation可改善其作为人类免疫缺陷病毒1型进入抑制剂的特性
许多人类蛋白质都有潜力被开发为治疗剂。但是,直接施用天然蛋白质引起的副作用已大大减慢了蛋白质治疗剂向临床的扩展。翻译后修饰(PTM)可以改善蛋白质特性,但是由于存在巨大的知识空白,我们使用PTM产生更好的蛋白质疗法的能力受到很大限制。在这里,我们试图通过研究小型代表性趋化细胞因子RANTES的PTM来填补空白。RANTES可以与HIV-1竞争与受体CCR5的结合并刺激CCR5的内吞作用,从而抑制HIV-1的感染。不幸的是,RANTES可以诱导强烈的信号传导,导致严重的炎症副作用。我们采用化学生物学方法来研究翻译后修饰的RANTES作为HIV-1感染的安全抑制剂的潜力。我们合成并系统地测试了RANTES亚型的文库,它们具有抑制炎症信号传导和预防人类原发性细胞HIV-1感染的能力。通过这项研究,我们发现大多数糖基化变体具有降低的炎症相关特性,并鉴定出一种特殊的糖变体,即一种截短的RANTES,其含有与Ser4连接的Galβ1-3GalNAc二糖α,具有出众的综合特性:相对较高的HIV-1抑制能力,但炎症特性较弱。此外,我们的结果为观察到的RANTES性质的变化提供了结构基础。在一起
更新日期:2017-12-14
中文翻译:

RANTES的O-GalNAcylation可改善其作为人类免疫缺陷病毒1型进入抑制剂的特性
许多人类蛋白质都有潜力被开发为治疗剂。但是,直接施用天然蛋白质引起的副作用已大大减慢了蛋白质治疗剂向临床的扩展。翻译后修饰(PTM)可以改善蛋白质特性,但是由于存在巨大的知识空白,我们使用PTM产生更好的蛋白质疗法的能力受到很大限制。在这里,我们试图通过研究小型代表性趋化细胞因子RANTES的PTM来填补空白。RANTES可以与HIV-1竞争与受体CCR5的结合并刺激CCR5的内吞作用,从而抑制HIV-1的感染。不幸的是,RANTES可以诱导强烈的信号传导,导致严重的炎症副作用。我们采用化学生物学方法来研究翻译后修饰的RANTES作为HIV-1感染的安全抑制剂的潜力。我们合成并系统地测试了RANTES亚型的文库,它们具有抑制炎症信号传导和预防人类原发性细胞HIV-1感染的能力。通过这项研究,我们发现大多数糖基化变体具有降低的炎症相关特性,并鉴定出一种特殊的糖变体,即一种截短的RANTES,其含有与Ser4连接的Galβ1-3GalNAc二糖α,具有出众的综合特性:相对较高的HIV-1抑制能力,但炎症特性较弱。此外,我们的结果为观察到的RANTES性质的变化提供了结构基础。在一起









































 京公网安备 11010802027423号
京公网安备 11010802027423号