当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Drug–Drug Interactions between Atorvastatin and Dronedarone Mediated by Monomeric CYP3A4
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b01012 Ilia G. Denisov 1 , Javier L. Baylon 1 , Yelena V. Grinkova 1 , Emad Tajkhorshid 1 , Stephen G. Sligar 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b01012 Ilia G. Denisov 1 , Javier L. Baylon 1 , Yelena V. Grinkova 1 , Emad Tajkhorshid 1 , Stephen G. Sligar 1
Affiliation
|
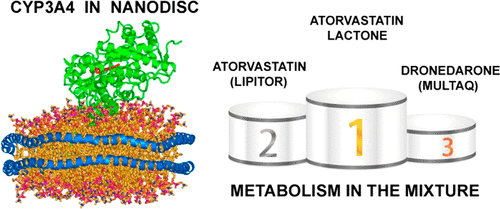
Heterotropic interactions between atorvastatin (ARVS) and dronedarone (DND) have been deciphered using global analysis of the results of binding and turnover experiments for pure drugs and their mixtures. The in vivo presence of atorvastatin lactone (ARVL) was explicitly taken into account by using pure ARVL in analogous experiments. Both ARVL and ARVS inhibit DND binding and metabolism, while a significantly higher affinity of CYP3A4 for ARVL makes the latter the main modulator of activity (effector) in this system. Molecular dynamics simulations reveal significantly different modes of interactions of DND and ARVL with the substrate binding pocket and with a peripheral allosteric site. Interactions of both substrates with residues F213 and F219 at the allosteric site play a critical role in the communication of conformational changes induced by effector binding to productive binding of the substrate at the catalytic site.
中文翻译:

CYP3A4单体介导的阿托伐他汀与决奈达隆之间的药物相互作用
阿托伐他汀(ARVS)和决奈达隆(DND)之间的异质相互作用已通过对纯药物及其混合物的结合和周转实验结果进行整体分析来破译。在体内在类似实验中,使用纯ARVL明确考虑了阿托伐他汀内酯(ARVL)的存在。ARVL和ARVS均抑制DND结合和代谢,而CYP3A4对ARVL的亲和力明显更高,后者成为该系统中活性(效应子)的主要调节剂。分子动力学模拟揭示了DND和ARVL与底物结合口袋和外围变构位点相互作用的显着不同模式。两种底物与别构位点上的残基F213和F219的相互作用在由效应子结合到底物在催化位点的有效结合所诱导的构象变化的传递中起关键作用。
更新日期:2017-12-14
中文翻译:

CYP3A4单体介导的阿托伐他汀与决奈达隆之间的药物相互作用
阿托伐他汀(ARVS)和决奈达隆(DND)之间的异质相互作用已通过对纯药物及其混合物的结合和周转实验结果进行整体分析来破译。在体内在类似实验中,使用纯ARVL明确考虑了阿托伐他汀内酯(ARVL)的存在。ARVL和ARVS均抑制DND结合和代谢,而CYP3A4对ARVL的亲和力明显更高,后者成为该系统中活性(效应子)的主要调节剂。分子动力学模拟揭示了DND和ARVL与底物结合口袋和外围变构位点相互作用的显着不同模式。两种底物与别构位点上的残基F213和F219的相互作用在由效应子结合到底物在催化位点的有效结合所诱导的构象变化的传递中起关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号