当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of HFC-134a as a Promoter of CO2 Hydrate: Phase Equilibrium, Dissociation Enthalpy and Kinetics
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.jced.7b00756 Hyunju Lee 1 , Chansu Park 2 , Eunkyung Lee 1 , Ju Dong Lee 3 , Yangdo Kim 4
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.jced.7b00756 Hyunju Lee 1 , Chansu Park 2 , Eunkyung Lee 1 , Ju Dong Lee 3 , Yangdo Kim 4
Affiliation
|
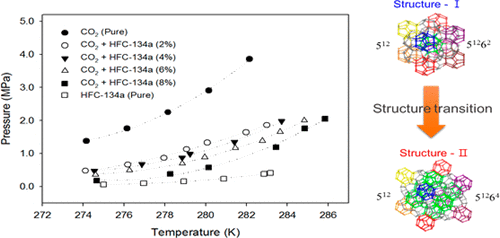
HFC-134a gas was investigated as a potential guest molecule to improve the thermodynamic conditions and formation rate for CO2 hydrate. In the phase equilibrium study, the equilibrium pressure of CO2 + HFC-134a was lower than that of pure CO2 gas, and the equilibrium pressure decreased gradually with increasing HFC-134a concentration. The dissociation enthalpy (ΔHd) was calculated using the Clausius–Clapeyron equation, and the ΔHd value also changed with increasing HFC-134a concentration. In particular, the ΔHd of 8 mol % HFC-134a-added CO2 hydrate was 143.2 kJ/mol, which was similar to that of pure HFC-134a (structure-II). In the kinetic study, the reactor was initially filled with CO2 + HFC-134a gas only and pure CO2 gas was then supplied as a source when the hydrate reaction proceeded. As a result, the formation rate of the HFC-134a mixture in the initial 2 min was faster than that of pure CO2. This was consistent with the gas chromatography results, which showed that HFC-134a occupies the cage at the beginning of hydrate formation. These results suggest that the addition of HFC-134a influences the CO2 hydrate thermodynamic equilibrium and kinetic characteristics.
中文翻译:

HFC-134a作为CO 2水合物促进剂的作用:相平衡,离解焓和动力学
研究了HFC-134a气体作为潜在的客体分子,以改善CO 2水合物的热力学条件和形成速率。在相平衡研究中,CO 2 + HFC-134a的平衡压力低于纯CO 2气体,并且随着HFC-134a浓度的增加,平衡压力逐渐降低。使用克劳修斯–克拉珀龙方程计算离解焓(ΔH d),并且ΔH d值也随着HFC-134a浓度的增加而变化。特别地,添加了8mol%的HFC-134a的CO 2的ΔH d水合物为143.2 kJ / mol,与纯HFC-134a(结构II)相似。在动力学研究中,反应器最初仅填充有CO 2 + HFC-134a气体,然后在水合物反应进行时,将纯CO 2气体作为原料提供。结果,在最初的2分钟内,HFC-134a混合物的形成速度要比纯CO 2的形成速度快。这与气相色谱结果一致,后者显示HFC-134a在水合物形成开始时就占据了笼子。这些结果表明,HFC-134a的添加会影响CO 2水合物的热力学平衡和动力学特性。
更新日期:2017-12-05
中文翻译:

HFC-134a作为CO 2水合物促进剂的作用:相平衡,离解焓和动力学
研究了HFC-134a气体作为潜在的客体分子,以改善CO 2水合物的热力学条件和形成速率。在相平衡研究中,CO 2 + HFC-134a的平衡压力低于纯CO 2气体,并且随着HFC-134a浓度的增加,平衡压力逐渐降低。使用克劳修斯–克拉珀龙方程计算离解焓(ΔH d),并且ΔH d值也随着HFC-134a浓度的增加而变化。特别地,添加了8mol%的HFC-134a的CO 2的ΔH d水合物为143.2 kJ / mol,与纯HFC-134a(结构II)相似。在动力学研究中,反应器最初仅填充有CO 2 + HFC-134a气体,然后在水合物反应进行时,将纯CO 2气体作为原料提供。结果,在最初的2分钟内,HFC-134a混合物的形成速度要比纯CO 2的形成速度快。这与气相色谱结果一致,后者显示HFC-134a在水合物形成开始时就占据了笼子。这些结果表明,HFC-134a的添加会影响CO 2水合物的热力学平衡和动力学特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号