当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor Pressure Osmometry, Volumetry, and Compressibility Properties for Solutions of Several Imidazolium Based Ionic Liquids in (Glycine + Water) Solutions
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.jced.7b00297 Sajjad Noshadi 1 , Rahmat Sadeghi 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.jced.7b00297 Sajjad Noshadi 1 , Rahmat Sadeghi 1
Affiliation
|
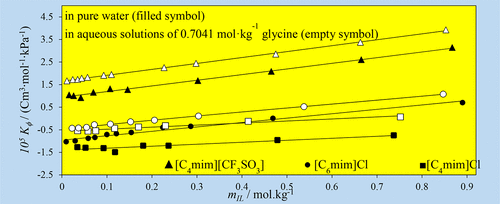
In this study, the effect of glycine on the thermodynamic properties of aqueous solutions containing ionic liquids 1-butyl-3-methylimidazolium chloride, [C4mim]Cl, 1-hexyl-3-methylimidazolium chloride, [C6mim]Cl, 1-butyl-3-methyl-imidazolium trifluoromethanesulfonate, [C4mim][CF3SO3], has been studied. For this purpose, first with help of the vapor pressure osmometery (VPO) method the water activity measurements have been performed on the ternary aqueous systems of (glycine + [C4mim]Cl, [C6mim]Cl, and [C4mim][CF3SO3]) at T = 308.15 K. The influence of the IL nature on the vapor–liquid equilibria behavior of the investigated systems has been studied. The VPO measurements of these solutions show that due to the unfavorable IL-glycine interactions, the negative deviations from the semi-ideal state and in conclude the soluting-out effects have been observed in these solutions. The soluting-out phenomena appears by aqueous biphasic system formation in the case of aqueous [C4mim][CF3SO3] + glycine system and precipitation of the glycine from the solution in the case of ternary [C4mim]Cl/[C6mim]Cl + glycine + water systems. In the second part of this work, density and sound velocity of solutions of the ILs in pure water and aqueous glycine solutions have been measured at four different temperatures. These data were used to obtain the apparent molar volume, Vϕ, isentropic compressibility, Kϕ, and those in the infinite dilution.
中文翻译:

(甘氨酸+水)溶液中几种咪唑基离子液体溶液的蒸气压渗透压测定法,体积法和可压缩性
在这项研究中,甘氨酸对含有离子液体1-丁基-3-甲基咪唑氯化物,[C 4 mim] Cl,1-己基-3-甲基咪唑氯化物,[C 6 mim] Cl,已经研究了[C 4 mim] [CF 3 SO 3 ]的1-丁基-3-甲基咪唑鎓三氟甲磺酸盐。为此,首先借助蒸气压渗透计(VPO)方法对(甘氨酸+ [C 4 mim] Cl,[C 6 mim] Cl和[C 4 mim] [CF 3 SO 3 ])在T= 308.15K。已研究了IL性质对所研究系统的气液平衡行为的影响。这些溶液的VPO测量结果表明,由于不利的IL-甘氨酸相互作用,导致从半理想状态产生负偏差,并最终在这些溶液中观察到溶出作用。在[C 4 mim] [CF 3 SO 3 ] +甘氨酸水溶液的情况下,水溶液双相体系的形成和在[C 4 mim] Cl /三元溶液的情况下,甘氨酸从溶液中的沉淀而出现溶出现象。[C 6mim] Cl +甘氨酸+水系统。在这项工作的第二部分中,已经在四个不同的温度下测量了纯净水和甘氨酸水溶液中IL溶液的密度和声速。这些数据被用于获得表观摩尔体积,V φ,等熵压缩,ķ φ,和那些在无限稀释。
更新日期:2017-12-05
中文翻译:

(甘氨酸+水)溶液中几种咪唑基离子液体溶液的蒸气压渗透压测定法,体积法和可压缩性
在这项研究中,甘氨酸对含有离子液体1-丁基-3-甲基咪唑氯化物,[C 4 mim] Cl,1-己基-3-甲基咪唑氯化物,[C 6 mim] Cl,已经研究了[C 4 mim] [CF 3 SO 3 ]的1-丁基-3-甲基咪唑鎓三氟甲磺酸盐。为此,首先借助蒸气压渗透计(VPO)方法对(甘氨酸+ [C 4 mim] Cl,[C 6 mim] Cl和[C 4 mim] [CF 3 SO 3 ])在T= 308.15K。已研究了IL性质对所研究系统的气液平衡行为的影响。这些溶液的VPO测量结果表明,由于不利的IL-甘氨酸相互作用,导致从半理想状态产生负偏差,并最终在这些溶液中观察到溶出作用。在[C 4 mim] [CF 3 SO 3 ] +甘氨酸水溶液的情况下,水溶液双相体系的形成和在[C 4 mim] Cl /三元溶液的情况下,甘氨酸从溶液中的沉淀而出现溶出现象。[C 6mim] Cl +甘氨酸+水系统。在这项工作的第二部分中,已经在四个不同的温度下测量了纯净水和甘氨酸水溶液中IL溶液的密度和声速。这些数据被用于获得表观摩尔体积,V φ,等熵压缩,ķ φ,和那些在无限稀释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号