当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterogeneous Oxidation of Particulate Methanesulfonic Acid by the Hydroxyl Radical: Kinetics and Atmospheric Implications
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00114 Emma L. Mungall 1 , Jenny P. S. Wong 1, 2 , Jonathan P. D. Abbatt 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00114 Emma L. Mungall 1 , Jenny P. S. Wong 1, 2 , Jonathan P. D. Abbatt 1
Affiliation
|
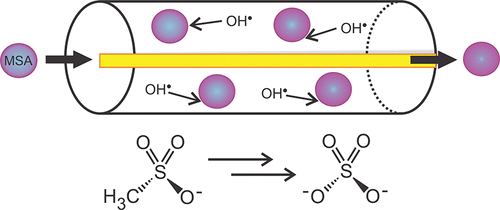
Dimethyl sulfide (DMS) is a major source of sulfur to the marine boundary layer (MBL), and methanesulfonic acid (MSA) is one of its two main final oxidation products. MSA can participate in the nucleation and growth of aerosol particles, thereby affecting clouds and climate, and is used as a tracer of biological sulfur inputs. Unlike MSA, the other major oxidation product of DMS, sulfate, has several other sources, including volcanic and anthropogenic inputs. As a result, MSA to non-sea-salt sulfate (nss-sulfate) ratios are often used as proxies for biological activity; i.e., the MSA to nss-sulfate ratio in aerosol particles is used to estimate the marine biological contribution to nss-sulfate in the MBL. We present here a determination of the reactive uptake coefficient, γ, for the heterogeneous oxidation of MSA by hydroxyl radicals within deliquesced ammonium sulfate aerosol particles with an MSA mass fraction of 0.16 (a typical marine value) at room temperature. We find γ = 0.05 ± 0.03. For high ambient gas-phase concentrations of the hydroxyl radical, this uptake coefficient corresponds to an estimated lifetime against heterogeneous oxidation of only a few days for MSA in MBL aerosol particles. Significantly, for typical gas-phase and condensed concentrations of the hydroxyl radical, the lifetime estimated here for heterogeneous oxidation is shorter than that for condensed-phase oxidation of MSA. This finding should be taken into consideration when using MSA to nss-sulfate ratios as tracers for DMS and biological activity, especially for air masses that have been exposed to considerable photochemical oxidation.
中文翻译:

羟基自由基对甲烷磺酸的异质氧化作用:动力学和大气意义
二甲基硫醚(DMS)是海洋边界层(MBL)的主要硫源,而甲磺酸(MSA)是其两个主要最终氧化产物之一。MSA可以参与气溶胶颗粒的成核和生长,从而影响云和气候,并被用作生物硫输入的示踪剂。与MSA不同,DMS的另一主要氧化产物硫酸盐具有其他多种来源,包括火山和人为输入。结果,MSA与非海盐硫酸盐(nss-硫酸盐)之比通常被用作生物活性的代表。即,气溶胶颗粒中MSA与nss-硫酸盐的比率用于估算MBL中海洋生物对nss-硫酸盐的贡献。我们在这里给出反应吸收系数γ的确定 用于在室温下将潮解性硫酸铵气溶胶颗粒中MSA质量分数为0.16(典型海洋值)的MSA羟基自由基异构化氧化MSA。我们发现γ= 0.05±0.03。对于较高的环境气相羟基自由基浓度,该吸收系数对应于MBL气溶胶颗粒中MSA对抗异质氧化仅几天的估计寿命。显着地,对于典型的气相和浓缩浓度的羟基自由基,此处估算的非均相氧化寿命比MSA的冷凝相氧化寿命短。当使用MSA对nss-硫酸盐的比率作为DMS和生物活性的示踪剂时,尤其是对于已暴露于大量光化学氧化的空气质量时,应考虑到这一发现。
更新日期:2017-12-19
中文翻译:

羟基自由基对甲烷磺酸的异质氧化作用:动力学和大气意义
二甲基硫醚(DMS)是海洋边界层(MBL)的主要硫源,而甲磺酸(MSA)是其两个主要最终氧化产物之一。MSA可以参与气溶胶颗粒的成核和生长,从而影响云和气候,并被用作生物硫输入的示踪剂。与MSA不同,DMS的另一主要氧化产物硫酸盐具有其他多种来源,包括火山和人为输入。结果,MSA与非海盐硫酸盐(nss-硫酸盐)之比通常被用作生物活性的代表。即,气溶胶颗粒中MSA与nss-硫酸盐的比率用于估算MBL中海洋生物对nss-硫酸盐的贡献。我们在这里给出反应吸收系数γ的确定 用于在室温下将潮解性硫酸铵气溶胶颗粒中MSA质量分数为0.16(典型海洋值)的MSA羟基自由基异构化氧化MSA。我们发现γ= 0.05±0.03。对于较高的环境气相羟基自由基浓度,该吸收系数对应于MBL气溶胶颗粒中MSA对抗异质氧化仅几天的估计寿命。显着地,对于典型的气相和浓缩浓度的羟基自由基,此处估算的非均相氧化寿命比MSA的冷凝相氧化寿命短。当使用MSA对nss-硫酸盐的比率作为DMS和生物活性的示踪剂时,尤其是对于已暴露于大量光化学氧化的空气质量时,应考虑到这一发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号