Immunity ( IF 25.5 ) Pub Date : 2017-12-05 , DOI: 10.1016/j.immuni.2017.11.003 Jie Wu , Hedong Zhang , Xiaomin Shi , Xiang Xiao , Yihui Fan , Laurie J. Minze , Jin Wang , Rafik M. Ghobrial , Jiahong Xia , Roger Sciammas , Xian C. Li , Wenhao Chen
|
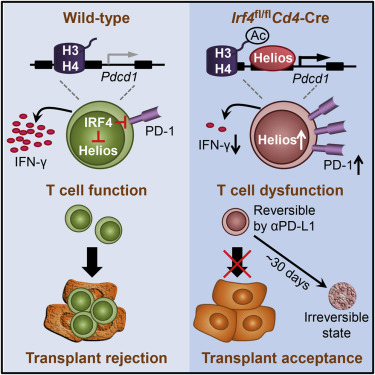
CD4+ T cells orchestrate immune responses and destruction of allogeneic organ transplants, but how this process is regulated on a transcriptional level remains unclear. Here, we demonstrated that interferon regulatory factor 4 (IRF4) was a key transcriptional determinant controlling T cell responses during transplantation. IRF4 deletion in mice resulted in progressive establishment of CD4+ T cell dysfunction and long-term allograft survival. Mechanistically, IRF4 repressed PD-1, Helios, and other molecules associated with T cell dysfunction. In the absence of IRF4, chromatin accessibility and binding of Helios at PD-1 cis-regulatory elements were increased, resulting in enhanced PD-1 expression and CD4+ T cell dysfunction. The dysfunctional state of Irf4-deficient T cells was initially reversible by PD-1 ligand blockade, but it progressively developed into an irreversible state. Hence, IRF4 controls a core regulatory circuit of CD4+ T cell dysfunction, and targeting IRF4 represents a potential therapeutic strategy for achieving transplant acceptance.
中文翻译:

转录因子IRF4的消除通过驱动同种异体CD4 + T细胞功能障碍促进移植接受。
CD4 + T细胞可以协调免疫应答和同种异体器官移植的破坏,但尚不清楚该过程在转录水平上如何调控。在这里,我们证明了干扰素调节因子4(IRF4)是移植过程中控制T细胞反应的关键转录决定因素。小鼠中IRF4缺失导致CD4 + T细胞功能障碍的逐步建立和同种异体移植的长期存活。从机制上讲,IRF4抑制PD-1,Helios和其他与T细胞功能障碍相关的分子。在没有IRF4的情况下,PD-1顺式调控元件的染色质可及性和Helios的结合增加,导致PD-1表达和CD4 +增强。T细胞功能障碍。Irf4缺陷型T细胞的功能障碍状态最初可通过PD-1配体阻断来逆转,但逐渐发展为不可逆状态。因此,IRF4控制着CD4 + T细胞功能障碍的核心调节回路,靶向IRF4代表了实现移植接受的潜在治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号