Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial.
The Lancet ( IF 98.4 ) Pub Date : 2018-01-01 , DOI: 10.1016/s1470-2045(17)30900-2 Manish R Patel 1 , John Ellerton 2 , Jeffrey R Infante 3 , Manish Agrawal 4 , Michael Gordon 5 , Raid Aljumaily 6 , Carolyn D Britten 7 , Luc Dirix 8 , Keun-Wook Lee 9 , Mathew Taylor 10 , Patrick Schöffski 11 , Ding Wang 12 , Alain Ravaud 13 , Arnold B Gelb 14 , Junyuan Xiong 14 , Galit Rosen 14 , James L Gulley 15 , Andrea B Apolo 16
The Lancet ( IF 98.4 ) Pub Date : 2018-01-01 , DOI: 10.1016/s1470-2045(17)30900-2 Manish R Patel 1 , John Ellerton 2 , Jeffrey R Infante 3 , Manish Agrawal 4 , Michael Gordon 5 , Raid Aljumaily 6 , Carolyn D Britten 7 , Luc Dirix 8 , Keun-Wook Lee 9 , Mathew Taylor 10 , Patrick Schöffski 11 , Ding Wang 12 , Alain Ravaud 13 , Arnold B Gelb 14 , Junyuan Xiong 14 , Galit Rosen 14 , James L Gulley 15 , Andrea B Apolo 16
Affiliation
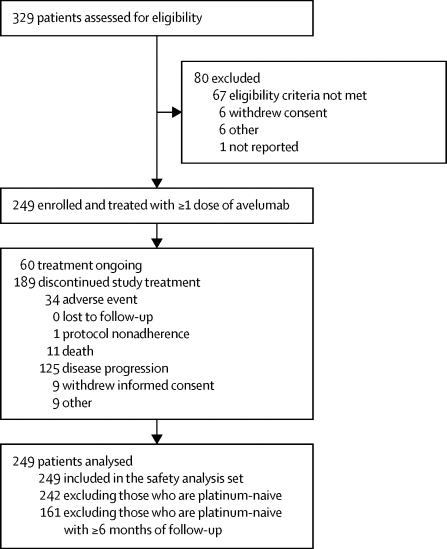
|
The approval of anti-programmed death ligand 1 (PD-L1) and anti-programmed death 1 agents has expanded treatment options for patients with locally advanced or metastatic urothelial carcinoma. Avelumab, a human monoclonal anti-PD-L1 antibody, has shown promising antitumour activity and safety in this disease. We aimed to assess the safety profile in patients (both post-platinum therapy and cisplatin-naive) treated with avelumab and to assess antitumour activity of this drug in post-platinum patients.
中文翻译:

铂类失败后转移性尿路上皮癌(JAVELIN 实体瘤)中的 Avelumab:来自开放标签、1 期试验的两个扩展队列的汇总结果。
抗程序性死亡配体 1 (PD-L1) 和抗程序性死亡 1 药物的批准扩大了局部晚期或转移性尿路上皮癌患者的治疗选择。Avelumab 是一种人类单克隆抗 PD-L1 抗体,在这种疾病中显示出有希望的抗肿瘤活性和安全性。我们旨在评估接受 avelumab 治疗的患者(铂类治疗后和顺铂初治)的安全性,并评估该药物在铂类后患者中的抗肿瘤活性。
更新日期:2017-12-31
中文翻译:

铂类失败后转移性尿路上皮癌(JAVELIN 实体瘤)中的 Avelumab:来自开放标签、1 期试验的两个扩展队列的汇总结果。
抗程序性死亡配体 1 (PD-L1) 和抗程序性死亡 1 药物的批准扩大了局部晚期或转移性尿路上皮癌患者的治疗选择。Avelumab 是一种人类单克隆抗 PD-L1 抗体,在这种疾病中显示出有希望的抗肿瘤活性和安全性。我们旨在评估接受 avelumab 治疗的患者(铂类治疗后和顺铂初治)的安全性,并评估该药物在铂类后患者中的抗肿瘤活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号