当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Borazine‐CF3− Adducts for Rapid, Room Temperature, and Broad Scope Trifluoromethylation
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711316 Jacob B. Geri 1 , Michael M. Wade Wolfe 1 , Nathaniel K. Szymczak 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711316 Jacob B. Geri 1 , Michael M. Wade Wolfe 1 , Nathaniel K. Szymczak 1
Affiliation
|
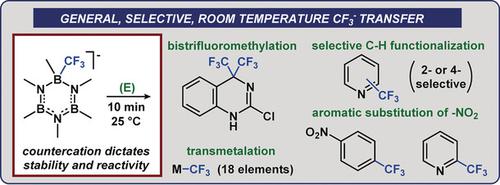
A fluoroform‐derived borazine CF3− transfer reagent is used to effect rapid nucleophilic reactions in the absence of additives, within minutes at 25 °C. Inorganic electrophiles spanning seven groups of the periodic table can be trifluoromethylated in high yield, including transition metals used for catalytic trifluoromethylation. Organic electrophiles included (hetero)arenes, enabling C−H and C−X trifluoromethylation reactions. Mechanistic analysis supports a dissociative mechanism for CF3− transfer, and cation modification afforded a reagent with enhanced stability.
中文翻译:

用于快速,室温和大范围三氟甲基化反应的Borazine-CF3-加合物
甲三氟甲烷衍生的环硼氮烷CF 3 -转移试剂在25用来快速作用的亲核反应在不存在添加剂时,在几分钟内℃。可以高产率地将跨越周期表的七个基团的无机亲电试剂三氟甲基化,包括用于催化三氟甲基化的过渡金属。有机亲电试剂包括(杂)芳烃,可实现CH和CX三氟甲基化反应。机理分析支持CF解离机构3 -转移,和阳离子改性,得到具有增强的稳定性的试剂。
更新日期:2018-01-09
中文翻译:

用于快速,室温和大范围三氟甲基化反应的Borazine-CF3-加合物
甲三氟甲烷衍生的环硼氮烷CF 3 -转移试剂在25用来快速作用的亲核反应在不存在添加剂时,在几分钟内℃。可以高产率地将跨越周期表的七个基团的无机亲电试剂三氟甲基化,包括用于催化三氟甲基化的过渡金属。有机亲电试剂包括(杂)芳烃,可实现CH和CX三氟甲基化反应。机理分析支持CF解离机构3 -转移,和阳离子改性,得到具有增强的稳定性的试剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号