当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Search for a photoinduced (site-selective) cleavage of the Ar–Cl bond in dichloroanisoles†
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1039/c7pp00372b Carlotta Raviola 1, 2, 3, 4 , Maurizio Fagnoni 1, 2, 3, 4
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1039/c7pp00372b Carlotta Raviola 1, 2, 3, 4 , Maurizio Fagnoni 1, 2, 3, 4
Affiliation
|
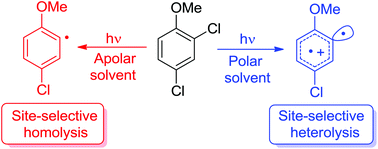
The site-selective cleavage of an Ar–X bond in polyhalogenated aromatics is an important tool in synthetic planning especially when more than one identical halogen atom is present. An alternative to the usual metal-catalyzed cleavage is represented by photochemistry although only a few examples have been reported. We then investigated the feasibility of the site-selective photodechlorination of some dichloroanisoles through a combined experimental and computational study. In the case of 2,4-dichloroanisole, selective detachment of the chlorine atom at the ortho position with respect to the OMe group was observed upon photohomolysis (in cyclohexane) or photoheterolysis (in MeOH) of the Ar–Cl bond. In the latter case, 5-chloro-2-methoxy-1,1′-biphenyl was exclusively formed upon reaction of the resulting phenyl cation with benzene. The substitution of an OH group for a OMe group was detrimental since a lower photoreactivity resulted with no improvement in the selectivity.
中文翻译:

在二氯茴香醚中寻找光诱导的Ar-Cl键的裂解(定点裂解)†
多卤代芳烃中Ar–X键的位点选择性裂解是合成规划中的重要工具,尤其是当存在多个相同的卤素原子时。尽管仅报道了几个例子,但光化学代表了通常的金属催化裂解的替代方法。然后,我们通过组合的实验和计算研究,研究了对某些二氯茴香醚进行位点选择性光脱氯的可行性。在2,4-二氯苯甲醚的情况下,邻位氯原子选择性分离相对于OMe基团的位置在Ar–Cl键的光均解(在环己烷中)或光杂解(在MeOH中)时观察到。在后一种情况下,仅在所得苯基阳离子与苯反应后形成5-氯-2-甲氧基-1,1'-联苯。OH基团代替OMe基团是有害的,因为导致较低的光反应性,而选择性没有改善。
更新日期:2017-12-04
中文翻译:

在二氯茴香醚中寻找光诱导的Ar-Cl键的裂解(定点裂解)†
多卤代芳烃中Ar–X键的位点选择性裂解是合成规划中的重要工具,尤其是当存在多个相同的卤素原子时。尽管仅报道了几个例子,但光化学代表了通常的金属催化裂解的替代方法。然后,我们通过组合的实验和计算研究,研究了对某些二氯茴香醚进行位点选择性光脱氯的可行性。在2,4-二氯苯甲醚的情况下,邻位氯原子选择性分离相对于OMe基团的位置在Ar–Cl键的光均解(在环己烷中)或光杂解(在MeOH中)时观察到。在后一种情况下,仅在所得苯基阳离子与苯反应后形成5-氯-2-甲氧基-1,1'-联苯。OH基团代替OMe基团是有害的,因为导致较低的光反应性,而选择性没有改善。











































 京公网安备 11010802027423号
京公网安备 11010802027423号