当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridacycles as Catalysts for the Autotandem Conversion of Nitriles into Amines by Hydrosilylation: Experimental Investigation and Scope
Organometallics ( IF 2.5 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.organomet.7b00749 Mustapha Hamdaoui 1 , Camille Desrousseaux 1 , Houda Habbita 1 , Jean-Pierre Djukic 1
Organometallics ( IF 2.5 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.organomet.7b00749 Mustapha Hamdaoui 1 , Camille Desrousseaux 1 , Houda Habbita 1 , Jean-Pierre Djukic 1
Affiliation
|
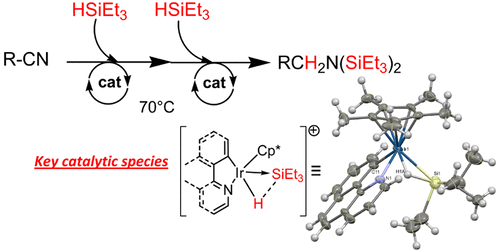
The set of iridacycles [{C,N}Cp*IrIII-Cl] ({C,N} = benzo[h]quinoline, dibenzo[f,h]quinoline) containing the (pentamethylcyclopentadienyl)iridium(III) unit were synthesized and derivatized into cations [{C,N}Cp*Ir-NCMe]+ associated with BArF-type anions. The latter salts were benchmarked for their potential catalytic properties toward HSiEt3 in a H2-releasing test reaction. The best-performing BArF-type salts demonstrated the capability to promote with a low catalytic load of ca. 0.5–1 mol % the autotandem hydrosilylation of acetonitrile, propionitrile, and a series of arylnitrile substrates. Mechanistic investigations confirmed the preliminary formation of a silane–iridacycle adduct by electrophilic and heterolytic activation of the Si–H bond. The molecular structure of a new example of such an adduct was resolved by X-ray diffraction analysis. Theoretical considerations support a donor–acceptor [{C,N}Cp*IrIII-H]→[SiEt3]+ ({C,N} = benzo[h]quinolinyl) formulation where the cationic silyl moiety acting as a Z ligand binds both Ir and H centers. Under the conditions of the catalysis, the latter adduct is assumed to transfer readily the electrophilic [SiEt3]+ moiety to the nucleophilic nitrile substrate to form a N-silylnitrilium cation and the neutral [{C,N}Cp*Ir-H]. The latter reduces the N-silylnitrilium into the corresponding N-silylimine, which undergoes further N-silylation and reduction to yield the final N,N-disilylamine. Under optimal conditions of low catalyst load (70 °C, 0.5 mol %) the autotandem hydrosilylation of arylnitriles produces the silylated amines in yields >80% in 24 h.
中文翻译:

Iridacycles作为通过氢化硅烷化将腈自动串联转化为胺的催化剂:实验研究和范围
合成了含有(五甲基环戊二烯基)铱(III)单元的一组iridacycles [{C,N} Cp * Ir III -Cl]({C,N} =苯并[ h ]喹啉,二苯并[ f,h ]喹啉)并衍生为与BArF型阴离子缔合的阳离子[{C,N} Cp * Ir-NCMe] +。后者的盐因其在H 2中对HSiEt 3的潜在催化性能而进行了基准测试-释放测试反应。表现最佳的BArF型盐显示出能够以低的催化负载量(大约)促进反应的能力。乙腈,丙腈和一系列芳腈底物的自动串联氢化硅烷化为0.5–1 mol%。机理研究证实,Si-H键的亲电和杂合活化可初步形成硅烷-铱环加合物。通过X射线衍射分析解析了这种加合物的新实例的分子结构。理论上的考虑支持了施主-受主[{C,N} Cp * Ir III -H]→[SiEt 3 ] +({C,N} =苯并[ h ]喹啉基)的配方,其中阳离子甲硅烷基部分充当Z配体结合Ir和H中心。在催化条件下,假定后者加成物易于将亲电子[SiEt 3 ] +部分转移至亲核腈底物上,形成N-甲硅烷基氮阳离子和中性[{C,N} Cp * Ir-H] 。后者将N-甲硅烷基腈还原成相应的N-甲硅烷基亚胺,其进一步进行N-甲硅烷基化和还原以产生最终的N,N-二甲硅烷基胺。在低催化剂负载(70°C,0.5 mol%)的最佳条件下,芳基腈的串联氢化硅烷化反应会在24小时内产生甲硅烷基化胺,收率> 80%。
更新日期:2017-12-04
中文翻译:

Iridacycles作为通过氢化硅烷化将腈自动串联转化为胺的催化剂:实验研究和范围
合成了含有(五甲基环戊二烯基)铱(III)单元的一组iridacycles [{C,N} Cp * Ir III -Cl]({C,N} =苯并[ h ]喹啉,二苯并[ f,h ]喹啉)并衍生为与BArF型阴离子缔合的阳离子[{C,N} Cp * Ir-NCMe] +。后者的盐因其在H 2中对HSiEt 3的潜在催化性能而进行了基准测试-释放测试反应。表现最佳的BArF型盐显示出能够以低的催化负载量(大约)促进反应的能力。乙腈,丙腈和一系列芳腈底物的自动串联氢化硅烷化为0.5–1 mol%。机理研究证实,Si-H键的亲电和杂合活化可初步形成硅烷-铱环加合物。通过X射线衍射分析解析了这种加合物的新实例的分子结构。理论上的考虑支持了施主-受主[{C,N} Cp * Ir III -H]→[SiEt 3 ] +({C,N} =苯并[ h ]喹啉基)的配方,其中阳离子甲硅烷基部分充当Z配体结合Ir和H中心。在催化条件下,假定后者加成物易于将亲电子[SiEt 3 ] +部分转移至亲核腈底物上,形成N-甲硅烷基氮阳离子和中性[{C,N} Cp * Ir-H] 。后者将N-甲硅烷基腈还原成相应的N-甲硅烷基亚胺,其进一步进行N-甲硅烷基化和还原以产生最终的N,N-二甲硅烷基胺。在低催化剂负载(70°C,0.5 mol%)的最佳条件下,芳基腈的串联氢化硅烷化反应会在24小时内产生甲硅烷基化胺,收率> 80%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号