Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2017-12-02 , DOI: 10.1016/j.bioorg.2017.12.006 Tarek Aboul-Fadl , Soliman S. Al-Hamad , Ehab A. Fouad
|
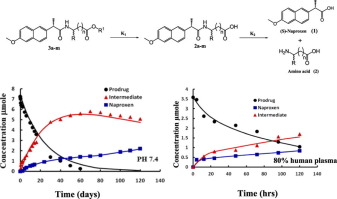
Naproxen (nap) is belonging to Non-steriodal anti-inflammatory drugs (NSAIDs) group of drugs that characterized by their free carboxylic group. The therapeutic activity of nap is usually accompanied by GI untoward side effects. Recently synthesized naproxen amides of some amino acid esters prodrugs to mask the free carboxylic group were reported. Those prodrugs showed a promising colorectal cancer chemopreventive activity. The current study aims to investigate the fate and hydrolysis of the prodrugs kinetically in different pH conditions, simulated gastric and intestinal fluids with pHs of 1.2, 5.5 and 7.4 in vitro at 37 °C. The effect of enzymes on the hydrolysis of prodrugs was also studied through incubation of these prodrugs at 37 °C in human plasma and rat liver homogenates. The pharmacokinetic parameters of selected prodrugs and the liberated nap were studied after oral and intraperitoneal administration in male wistar rats. The results showed the hydrolysis of naproxen amides of amino acid esters to nap through two steps first by degradation of the ester moiety to form the amide of nap with amino acid and the second was through the degradation of the amide link to liberate nap. The two reactions were followed and studied kinetically where K1 and K2 (rate constants of degradation) is reported. The hydrolysis of prodrugs was faster in liver homogenates than in plasma. The relative bioavailability of the liberated nap in vivo was higher in case of prodrug containing ethyl glycinate moiety than that occupied l-valine ethyl ester moiety. Each of nap. prodrugs containing ethyl glycinate and l-valine ethyl ester moieties appears promising in liberating nap, decreasing direct GI side effect and consequently their colorectal cancer chemopreventive activity.
中文翻译:

具有前景的大肠癌化学预防活性的某些氨基酸的萘普生酰胺的药代动力学研究
萘普生(nap)属于非杀菌抗炎药(NSAIDs)组,其特征在于其游离羧基。午睡的治疗活性通常伴有胃肠不良反应。据报道,最近合成的某些氨基酸酯前药的萘普生酰胺掩盖了游离的羧基。这些前药显示出有希望的结直肠癌化学预防活性。当前的研究旨在动力学地研究前药在不同pH条件,体外pH和pH为1.2、5.5和7.4的模拟胃液和肠液中的命运和水解情况。在37°C下。还通过在人血浆和大鼠肝匀浆中于37°C孵育这些前药来研究酶对前药水解的影响。在雄性Wistar大鼠中口服和腹膜内给药后,研究了所选前药和释放的午睡的药代动力学参数。结果表明,氨基酸酯的萘普生酰胺水解通过两个步骤进行水解,第一步是通过酯部分的降解以与氨基酸形成午睡的酰胺,第二步是通过酰胺键的降解以释放午睡。跟踪两个反应并进行动力学研究,其中K 1和K 2(降解速率常数)的报告。肝匀浆中前药的水解比血浆中的要快。在含有甘氨酸乙酯部分的前药的情况下,所释放的午睡在体内的相对生物利用度高于所占据的1-缬氨酸乙酯部分。每次小睡。含有甘氨酸乙酯和1-缬氨酸乙酯部分的前药在释放午睡,降低直接胃肠道副作用以及因此降低其对大肠癌的化学预防活性方面似乎很有希望。









































 京公网安备 11010802027423号
京公网安备 11010802027423号