Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2017-12-02 , DOI: 10.1016/j.bioorg.2017.11.019 Hany E.A. Ahmed , Saleh K. Ihmaid , Abdelsattar M. Omar , Ahmed M. Shehata , Heba S. Rateb , Mohammed F. Zayed , Sahar Ahmed , Mahmoud M. Elaasser
|
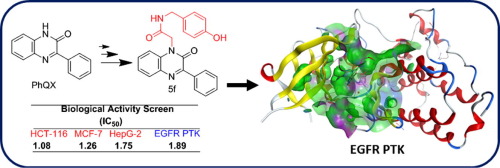
Fifteen new substituted N-2-(2-oxo-3-phenylquinoxalin-1(2H)-yl) acetamides 5a-f, 6a-f, and 8a-c were synthesized by reacting ethyl 2-(2-oxo-3-phenylquinoxalin-1(2H)-yl)acetate with various primary amines including benzylamines, sulfonamides, and amino acids. The in vitro antimicrobial screening of the target compounds was screened to assess their antibacterial and antifungal activity. As a result, seven compounds namely; 5a, 5c, 5d, 6a, 6c, 8b and 8c showed a promising broad spectrum antibacterial activity against both Gram-positive and Gram-negative strains. Among these, the analogs 5c and 6d were nearly as equiactive as ciprofloxacin drug. Meanwhile, four compounds namely; 5c, 6a, 6f and 8c exhibited appreciable antifungal activity with MIC values range 33–40 mg/mL comparable with clotrimazole (MIC 25 mg/mL). In addition, the anticancer effects of the synthesized compounds were evaluated against three cancer lines. The data obtained revealed the benzylamines and sulpha derivatives were the most active compounds especially 5f and 6f ones. Further EGFR enzymatic investigation was carried out for these most active compounds 5f and 6f resulting in inhibitory activity by 1.89 and 2.05 µM respectively. Docking simulation was performed as a trial to study the mechanisms and binding modes of these compounds toward the enzyme target, EGFR protein kinase enzyme. The results revealed good compounds placement in the active sites and stable interactions similar to the co-crystallized reference ligand. Collectively, the analogs 5f and 6f could be further utilized and optimized as good cytotoxic agents.
中文翻译:

新的亲脂性乙酰胺衍生物的设计,合成,分子对接,可提供潜在的抗癌药和抗菌剂
通过使2-(2-氧代-3-乙基)乙基合成十五个新的取代的N-2-(2-氧代-3-苯基喹喔啉-1(2H)-基)乙酰胺5a-f,6a-f和8a-c。苯基喹喔啉-1(2H)-基)乙酸酯与各种伯胺,包括苄胺,磺酰胺和氨基酸。筛选了目标化合物的体外抗菌筛选,以评估其抗菌和抗真菌活性。结果,七种化合物分别为:5a,5c,5d,6a,6c,8b和8c对革兰氏阳性和革兰氏阴性菌株均显示出有前景的广谱抗菌活性。其中,类似物5c和6d具有与环丙沙星药物同等的活性。同时,有四种化合物:5c,6a,6f和8c表现出明显的抗真菌活性,MIC值在33–40 mg / mL之间,与克霉唑(MIC 25 mg / mL)相当。另外,评估了合成化合物对三种癌症细胞系的抗癌作用。获得的数据表明,苄胺和磺胺衍生物是活性最高的化合物,尤其是5f和6f那些。对这些活性最高的化合物5f和6f进行了进一步的EGFR酶促研究,分别产生了1.89和2.05 µM的抑制活性。作为试验进行了对接模拟,以研究这些化合物对酶靶标EGFR蛋白激酶的机理和结合方式。结果表明,与共结晶的参考配体相似,化合物在活性位点中的位置良好,相互作用稳定。总的来说,类似物5f和6f可被进一步利用和优化为良好的细胞毒性剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号